Question: Use the data in the table below to answer this question. Given the reaction below and the reaction enthalpy (Hrxn), calculate the H f for
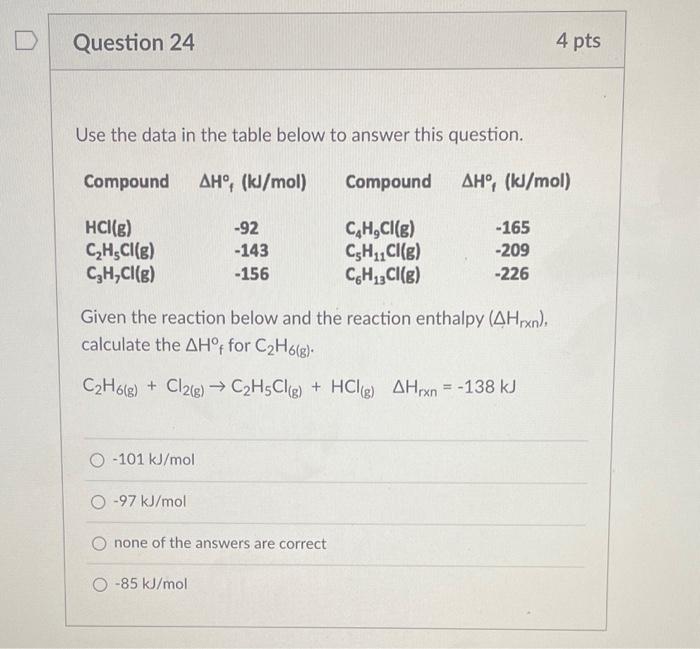
Use the data in the table below to answer this question. Given the reaction below and the reaction enthalpy (Hrxn), calculate the H f for C2H6(g). C2H6(g)+Cl2(g)C2H5Cl(g)+HCl(g)Hrxn=138kJ 101kJ/mol 97kJ/mol none of the answers are correct 85kJ/mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


