Question: Use the data table below to answer the following questions. so Substance Molar Mass (g/mol) 30.006 44.013 , (kJ/mol) 90.3 AGF (kJ/mol) p/molK)] 210.7 NO
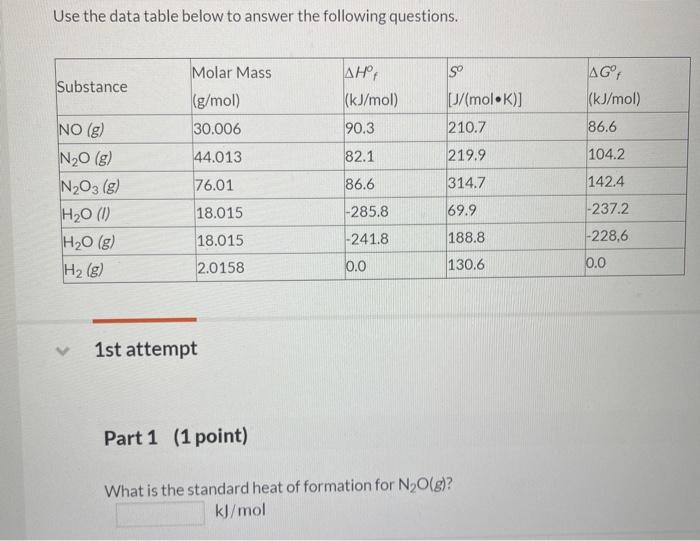
![Substance Molar Mass (g/mol) 30.006 44.013 , (kJ/mol) 90.3 AGF (kJ/mol) p/molK)]](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9203f66312_75166f9203f015d1.jpg)
Use the data table below to answer the following questions. so Substance Molar Mass (g/mol) 30.006 44.013 , (kJ/mol) 90.3 AGF (kJ/mol) p/molK)] 210.7 NO (g) 86.6 82.1 219.9 104.2 76.01 314.7 142.4 N20 (g) NO (g) H20 (0) H2O(g) H2(g) 86.6 -285.8 18.015 69.9 -237.2 -228,6 18.015 -241.8 188.8 2.0158 0.0 130.6 0.0 1st attempt Part 1 (1 point) What is the standard heat of formation for N2O(g)? kJ/mol Part 1 (1 point) What is the standard heat of formation for N2O(g)? kJ/mol Part 2 (1 point) What is the standard heat of formation for H2O(g)? kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


