Question: Use the References to access important values if needed for this question. Use the table 'Standard Reduction Potentials' located in the 'Tables', to predict if
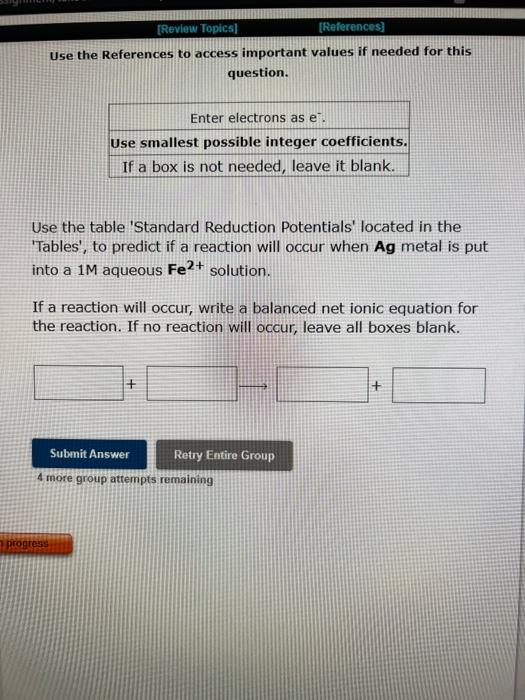
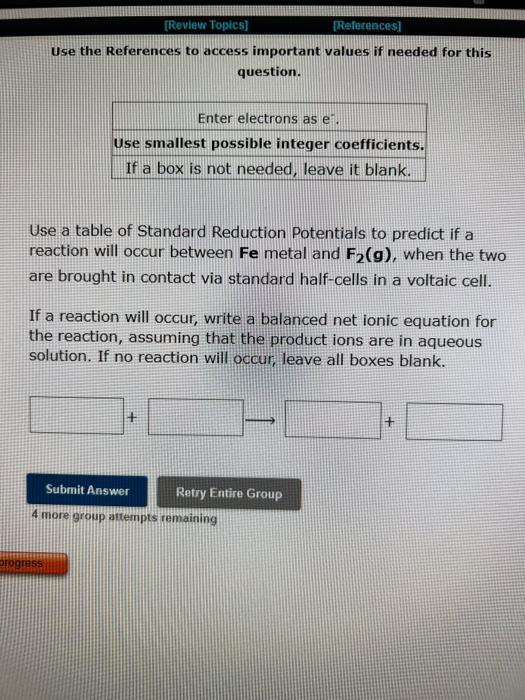
Use the References to access important values if needed for this question. Use the table 'Standard Reduction Potentials' located in the 'Tables', to predict if a reaction will occur when Ag metal is put into a 1M aqueous Fe2+ solution. If a reaction will occur, write a balanced net ionic equation for the reaction. If no reaction will occur, leave all boxes blank. 4 more group attempts remaining Use the References to access important values if needed for this question. Use a table of Standard Reduction Potentials to predict if a reaction will occur between Fe metal and F2(g), when the two are brought in contact via standard half-cells in a voltaic cell. If a reaction will occur, write a balanced net ionic equation for the reaction, assuming that the product ions are in aqueous solution. If no reaction will occur, leave all boxes blank. 4 more group artempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


