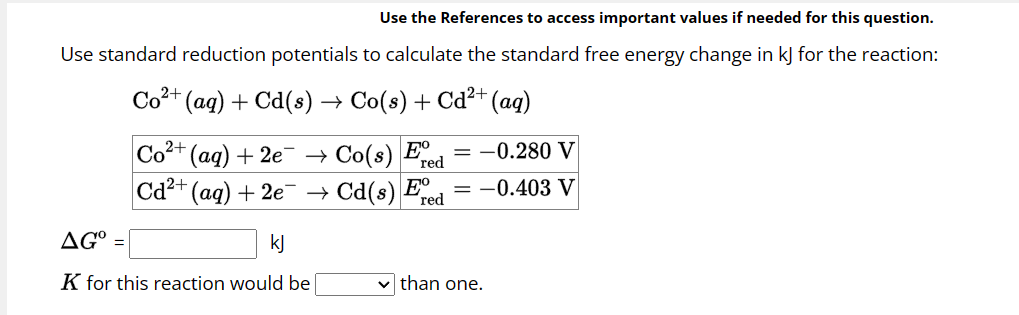
Question: Use the References to access important values if needed for this question. Use standard reduction potentials to calculate the standard free energy change in k
Use the References to access important values if needed for this question.
Use standard reduction potentials to calculate the standard free energy change in for the reaction:
for this reaction would be
than one.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


