Question: Use the References te access important values if needed for this question, 3ClO4+2Bi+3H2O3ClO3+2Bi(OH)3 In the above reaction, the oxidation state of chlorine changes from to
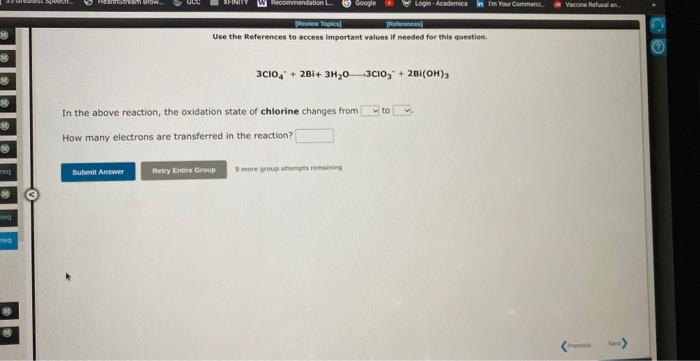
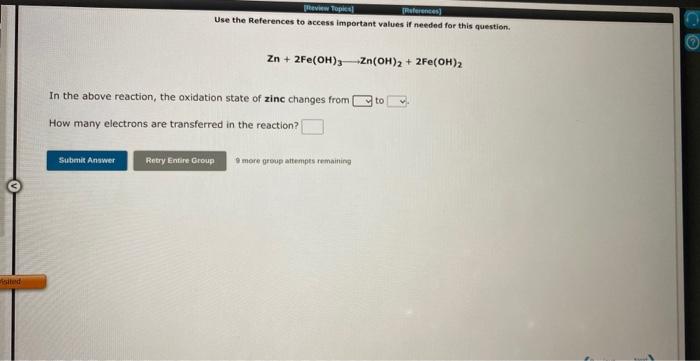
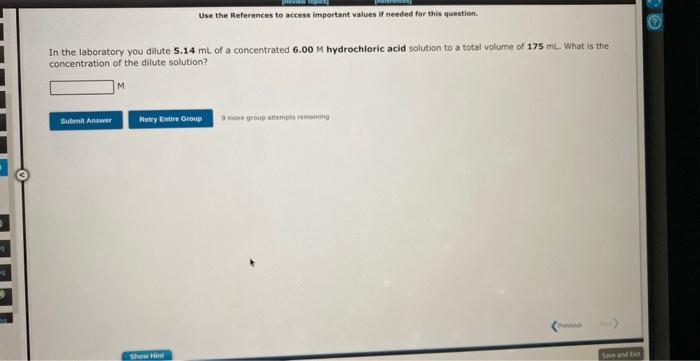
Use the References te access important values if needed for this question, 3ClO4+2Bi+3H2O3ClO3+2Bi(OH)3 In the above reaction, the oxidation state of chlorine changes from to How many electrons are transferred in the reaction? Use the References to access important values if needed for this question. Zn+2Fe(OH)3Zn(OH)2+2Fe(OH)2 In the above reaction, the oxidation state of zinc changes from to How many electrons are transferred in the reaction? In the laboratory you dilute 5.14mL of a concentrated 6.00M hydrochloric acid solution to a total volume of 175mL. What is the concentration of the dilute solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


