Question: Use the References to access important values if needed for this question. A sample of nitrogen gas occupies a volume of 7.98L at 55.0C and
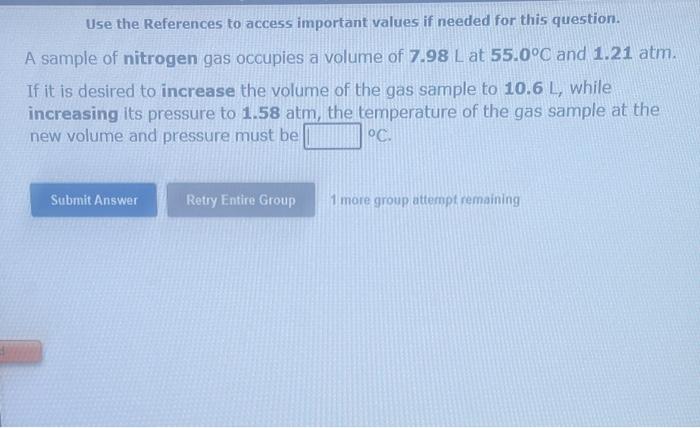
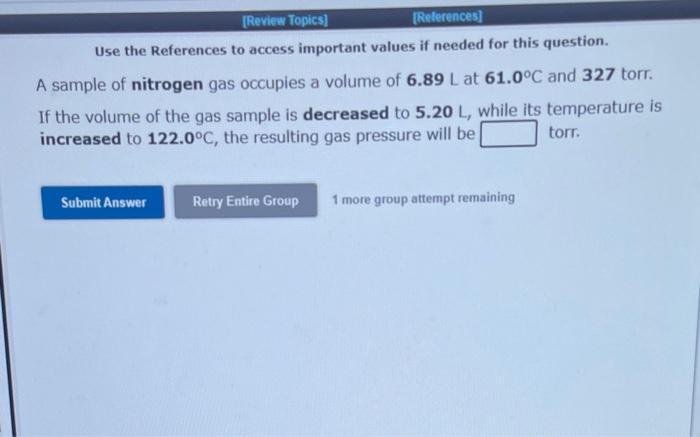
Use the References to access important values if needed for this question. A sample of nitrogen gas occupies a volume of 7.98L at 55.0C and 1.21atm. If it is desired to increase the volume of the gas sample to 10.6L, while increasing its pressure to 1.58atm, the temperature of the gas sample at the new volume and pressure must be oc. 1 more group attempt remaining Use the References to access important values if needed for this question. A sample of nitrogen gas occupies a volume of 6.89L at 61.0C and 327 torr. If the volume of the gas sample is decreased to 5.20L, while its temperature is increased to 122.0C, the resulting gas pressure will be torr. 1 more group attempt remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


