Question: Use the References to access important values if needed for this question. A sample of nitrogen gas at a pressure of 1.19 atm and a
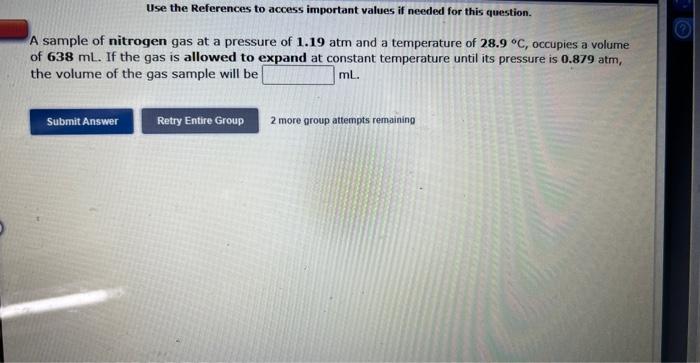
Use the References to access important values if needed for this question. A sample of nitrogen gas at a pressure of 1.19 atm and a temperature of 28.9C, occupies a volume of 638mL. If the gas is allowed to expand at constant temperature until its pressure is 0.879 atm, the volume of the gas sample will be mL. 2 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


