Question: Use the References to access important values if needed for this question. A mixture consisting of only aluminum chloride (AlCl3) and chromium(III) chloride (CrCl3) weighs
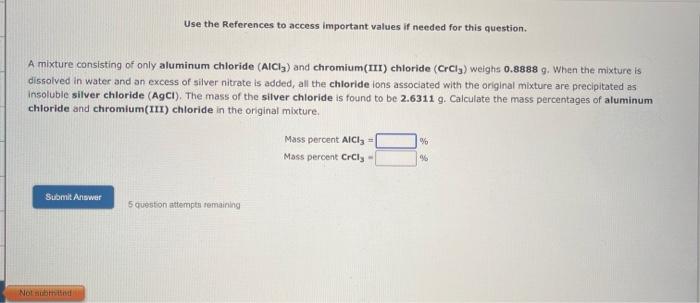
Use the References to access important values if needed for this question. A mixture consisting of only aluminum chloride (AlCl3) and chromium(III) chloride (CrCl3) weighs 0.8888 g. When the mixture is dissolved in water and an excess of silver nitrate is added, all the chloride ions associated with the original mixture are precipitated as insoluble silver chloride ( AgCi ). The mass of the silver chloride is found to be 2.6311g. Calculate the mass percentages of aluminum chloride and chromium(III) chloride in the original mixture. Mass percent AiCl3= Mass percent CrCl3=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


