Question: Use the References to access important values if needed for this question. A 0.282mol sample of an unknown gas contained in a 8.00L flask is


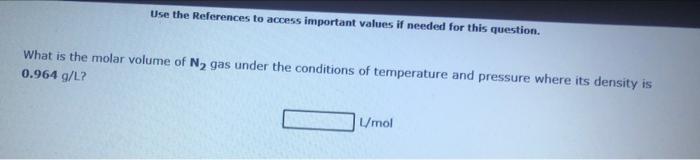
Use the References to access important values if needed for this question. A 0.282mol sample of an unknown gas contained in a 8.00L flask is found to have a density of 1.57g/L. The molecular weight of the unknown gas is g/mol. Use the References to access important values if needed for this question. A 0.309mol sample of Ar gas is contained in a 8.00L flask at room temperature and pressure. What is the density of the gas, in grams/liter, under these conditions? g/L Use the References to access important values if needed for this question. What is the molar volume of N2 gas under the conditions of temperature and pressure where its density is 0.964g/L ? Unol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


