Question: Use the References to access important values if needed for this question. Which of the following aqueous solutions are good buffer systems? 0.15M hydrocyanic acid
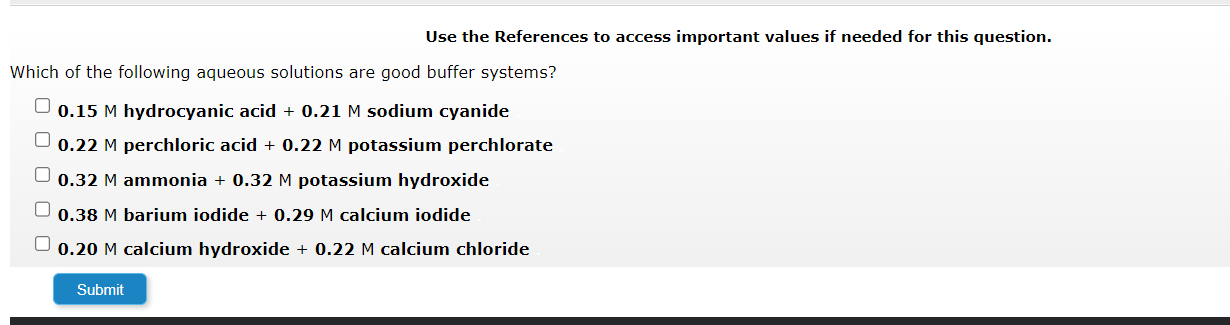
Use the References to access important values if needed for this question. Which of the following aqueous solutions are good buffer systems? 0.15M hydrocyanic acid +0.21M sodium cyanide 0.22M perchloric acid +0.22M potassium perchlorate 0.32M ammonia +0.32M potassium hydroxide 0.38M barium iodide +0.29M calcium iodide 0.20M calcium hydroxide +0.22M calcium chloride
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


