Question: Use the References to access important values if needed for this question. The gas phase decomposition of sulfuryl chloride at 600K SO2Cl2(g)SO2(g)+Cl2(g) is first order
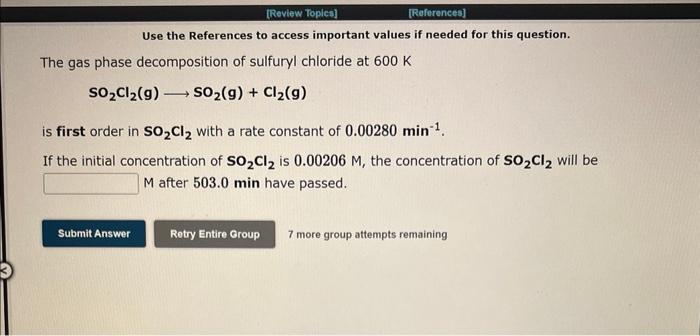

Use the References to access important values if needed for this question. The gas phase decomposition of sulfuryl chloride at 600K SO2Cl2(g)SO2(g)+Cl2(g) is first order in SO2Cl2 with a rate constant of 0.00280min1. If the initial concentration of SO2Cl2 is 0.00206M, the concentration of SO2Cl2 will be M after 503.0 min have passed. 7 more group attempts remaining Use the References to access important values if needed for this question. The conversion of methyl isonitrile to acetonitrile in the gas phase at 250C CH3NC(g)CH3CN(g) is first order in CH3NC with a rate constant of 0.00300s1. If the initial concentration of CH3NC is 0.117M, the concentration of CH3NC will be 0.0294M after 5 have passed. 7 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


