Question: Use the References to access important values if needed for this question. The gas phase reaction of iodine with silane takes place according to a
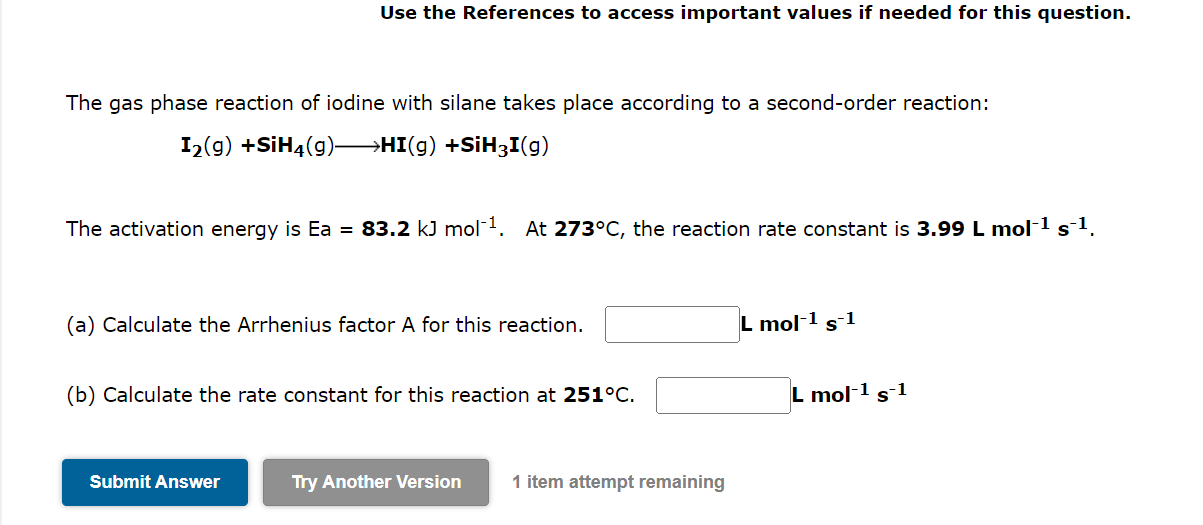
Use the References to access important values if needed for this question. The gas phase reaction of iodine with silane takes place according to a second-order reaction: I2(g)+SiH4(g)HI(g)+SiH3I(g) The activation energy is Ea=83.2kJmol1. At 273C, the reaction rate constant is 3.99Lmol1s1. (a) Calculate the Arrhenius factor A for this reaction. Lmol1s1 (b) Calculate the rate constant for this reaction at 251C.Lmol1s1 1 item attempt remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


