Question: Use the References to access important values if needed for this question; The boiling point of water is 100.0C at 1 atmosphere. How many grams
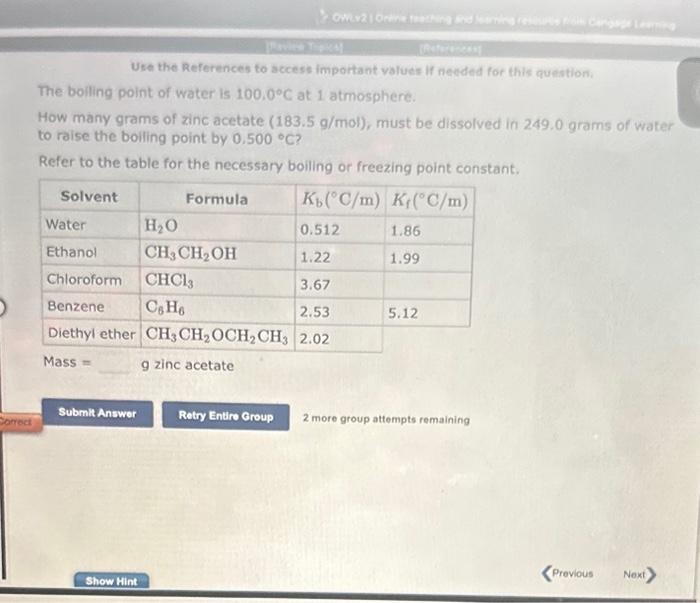
Use the References to access important values if needed for this question; The boiling point of water is 100.0C at 1 atmosphere. How many grams of zinc acetate (183.5 g/mol), must be dissolved in 249.0 grams of water to raise the boiling point by 0.500C ? Refer to the table for the necessary boiling or freezing point constant. Mass = 9 zinc acetate 2 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


