Question: Use the Relerences to access important values if needed for this question. In the laboratory, a student adds 60.0mL of water to 14.6mL of a
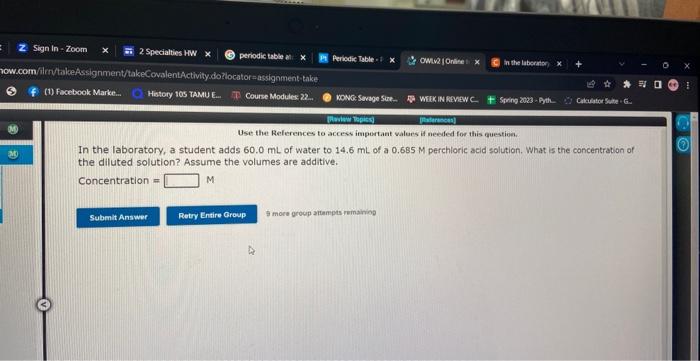
Use the Relerences to access important values if needed for this question. In the laboratory, a student adds 60.0mL of water to 14.6mL of a 0.685M perchloric acid solution. What is the concentration of the diluted solution? Assume the volumes are additive. Concentration = M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


