Question: Use the Roferences te access important valuns if needed for this queseon- The following information is given for lead at. satm; boiling point = 1740=C
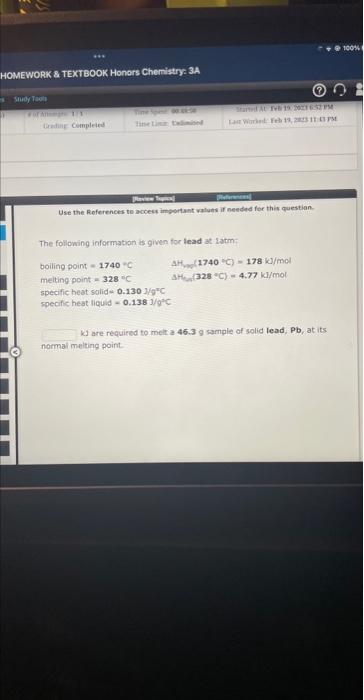
Use the Roferences te access important valuns if needed for this queseon- The following information is given for lead at. satm; boiling point = 1740=C AHf (1740cc)=178k//mol meltingpoint =328 " CSH(32c+C)=4.77kJ/mol specific beat solid =0.13.0.kgNC specific heat liquid =0.138j/q2C k. are required to melt a 46.3g sample of solid lead. Pb, at its. normal melting point
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


