Question: Use the values in Table 4-1 and Table 4-2 to solve the problems in this question. For these problems, assume that the values of
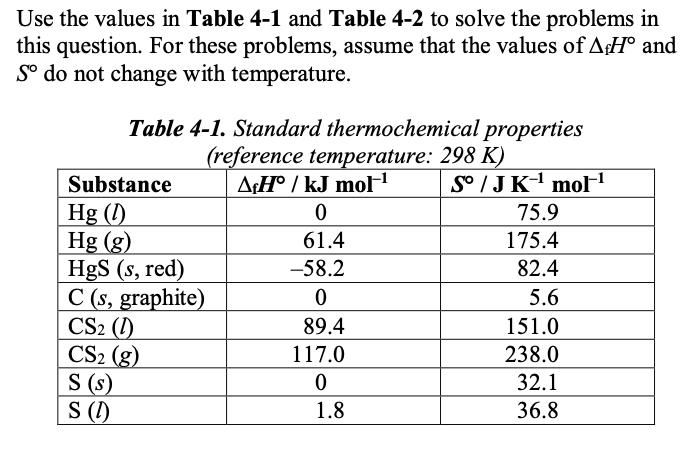
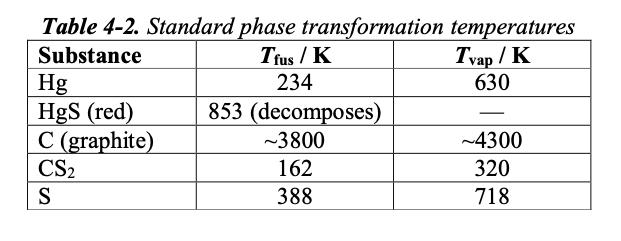

Use the values in Table 4-1 and Table 4-2 to solve the problems in this question. For these problems, assume that the values of AH and So do not change with temperature. Table 4-1. Standard thermochemical properties (reference temperature: 298 K) AH/kJ mol- So / J K mol- Substance Hg (1) Hg (g) HgS (s, red) C (s, graphite) CS2 (1) CS (g) S (s) S (1) 0 61.4 -58.2 0 89.4 117.0 0 1.8 75.9 175.4 82.4 5.6 151.0 238.0 32.1 36.8 Table 4-2. Standard phase transformation temperatures Substance Tfus / K 234 853 (decomposes) ~3800 162 388 Hg HgS (red) C (graphite) CS S Tvap/K 630 ~4300 320 718 (i) List which thermodynamic value can be determined from the intercept of a line on an Ellingham diagram with the y- axis at 0 K. List which thermodynamic value can be determined from the slope of a line on an Ellingham diagram. (iii) Explain why the slope of a line on an Ellingham diagram changes if the phase of the product or reactant changes to a gas.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


