Question: Use the van't Hoff factors in the table below to calculate each colligative property. Van't Hoff factors in aqueous solution the melting point of a
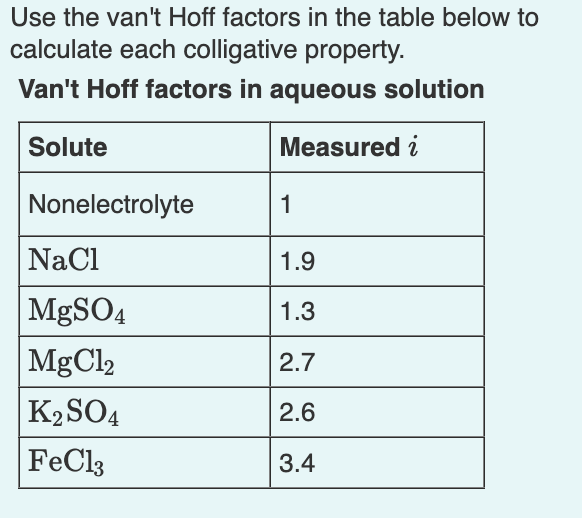
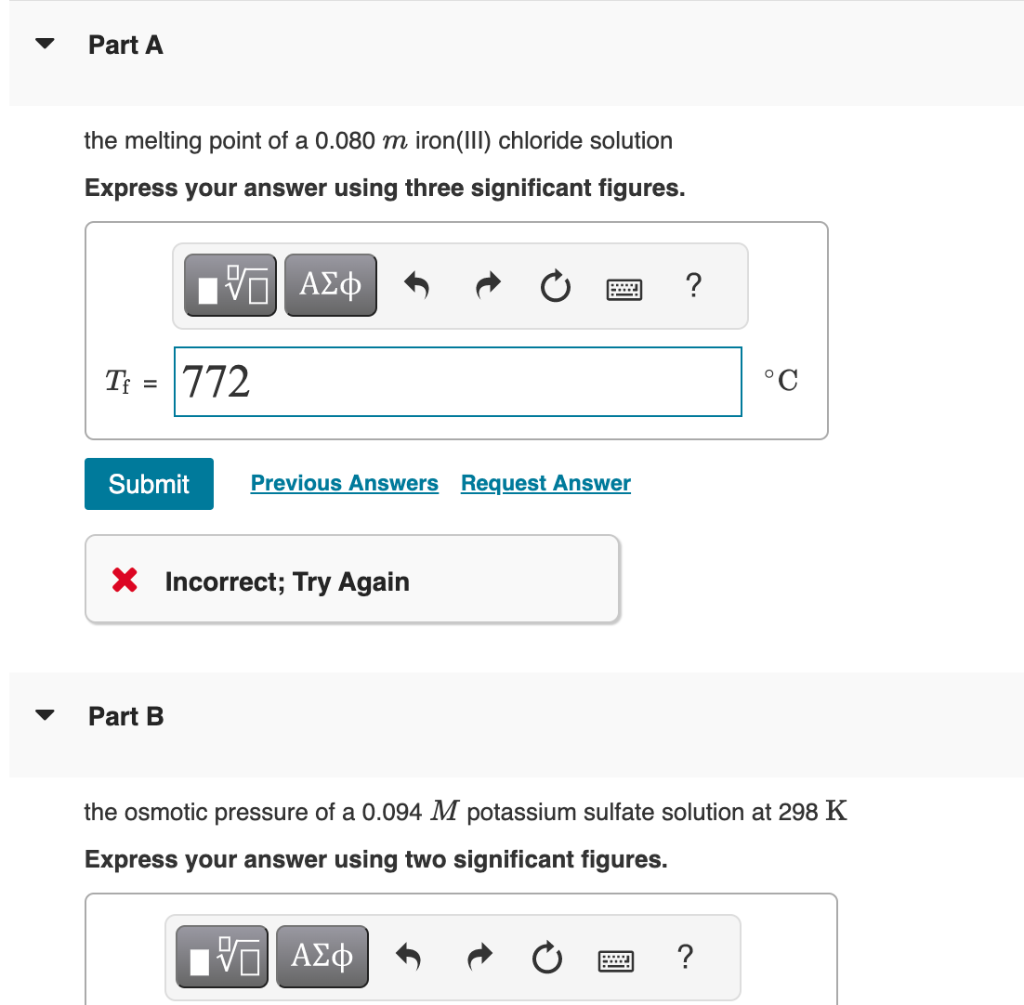
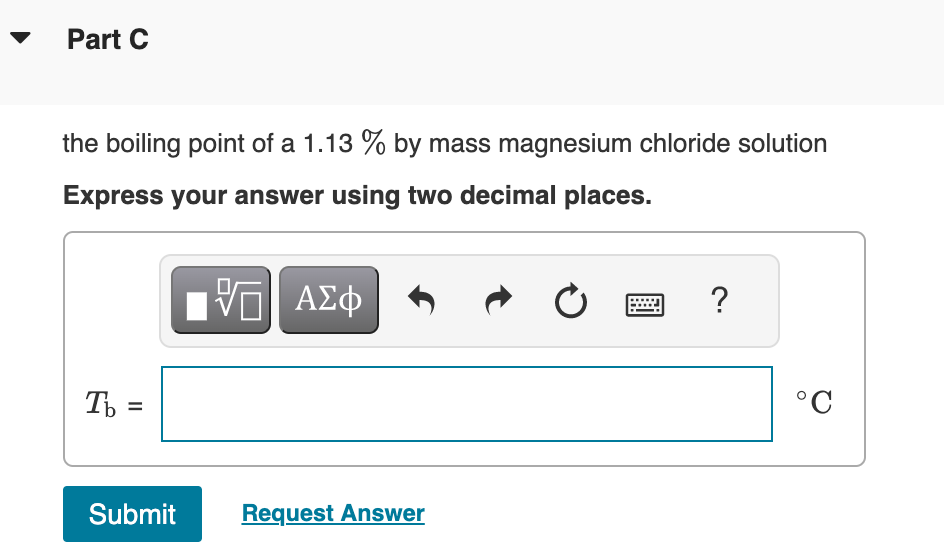
Use the van't Hoff factors in the table below to calculate each colligative property. Van't Hoff factors in aqueous solution the melting point of a 0.080m iron(III) chloride solution Express your answer using three significant figures. T C Part B the osmotic pressure of a 0.094M potassium sulfate solution at 298K Express your answer using two significant figures. the boiling point of a 1.13% by mass magnesium chloride solution Express your answer using two decimal places
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


