Question: use Untererences to access important values if needed for this question. In a study of the decomposition of hydrogen peroxide in dilute sodium hydroxide at
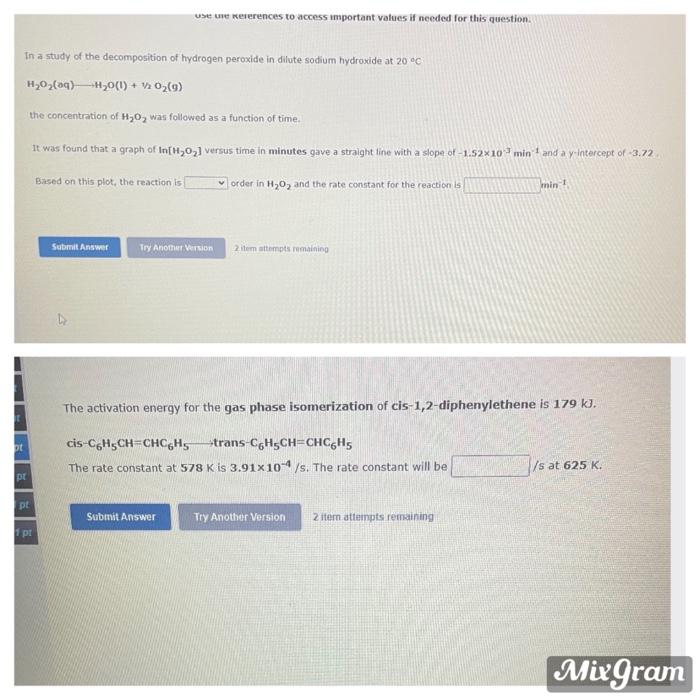
use Untererences to access important values if needed for this question. In a study of the decomposition of hydrogen peroxide in dilute sodium hydroxide at 20C H2O2(aq)-H20(1) + 12 02(9) the concentration of H,0, was followed as a function of time It was found that a graph of In[H,O,] versus time in minutes gave a straight line with a slope of -1.52x10min and a y intercept of -3.72 Based on this plot, the reaction is order in H20, and the rate constant for the reaction is min Submit Answer Try Another VISION 2 itemstompts remaining The activation energy for the gas phase isomerization of cis-1,2-diphenylethene is 179 KI. ot cis-C6H5CH=CHCGHS trans-C6H5CH=CHC6H5 The rate constant at 578 K is 3.91x10- /s: The rate constant will be Vs at 625 K. pl pl Submit Answer Try Another Version 2 iter attempts remaining pt Mixgram
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


