Question: Using a third order Newtons interpolating polynomial, estimate the composition of the vapor that is in equilibrium with liquid which contains 5 mole % ethanol
Using a third order Newtons interpolating polynomial, estimate the composition of the vapor that is in equilibrium with liquid which contains 5 mole % ethanol and 95 mole % water at 1 atm pressure. Use the data given below.
# Numerical Methods
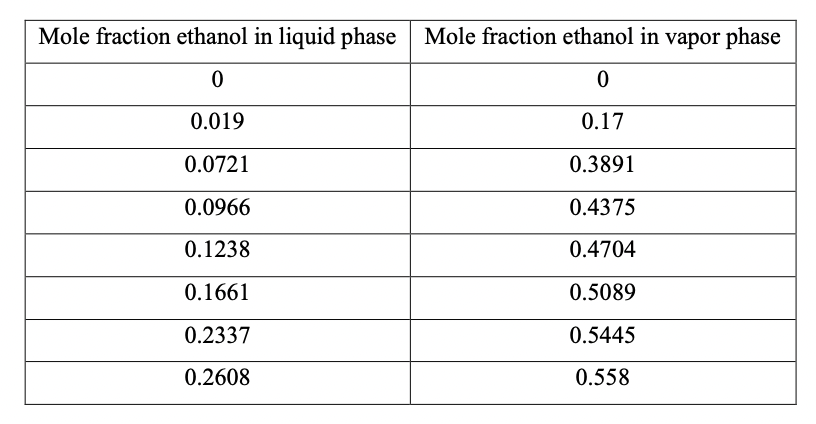
\begin{tabular}{|c|c|} \hline Mole fraction ethanol in liquid phase & Mole fraction ethanol in vapor phase \\ \hline 0 & 0 \\ \hline 0.019 & 0.17 \\ \hline 0.0721 & 0.3891 \\ \hline 0.0966 & 0.4375 \\ \hline 0.1238 & 0.4704 \\ \hline 0.1661 & 0.5089 \\ \hline 0.2337 & 0.5445 \\ \hline 0.2608 & 0.558 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


