Question: Using Appendix B, determine the entropy change values for the reactions. Add the values to your table. # Reaction 1 N2(g) +3 H2(g) AS
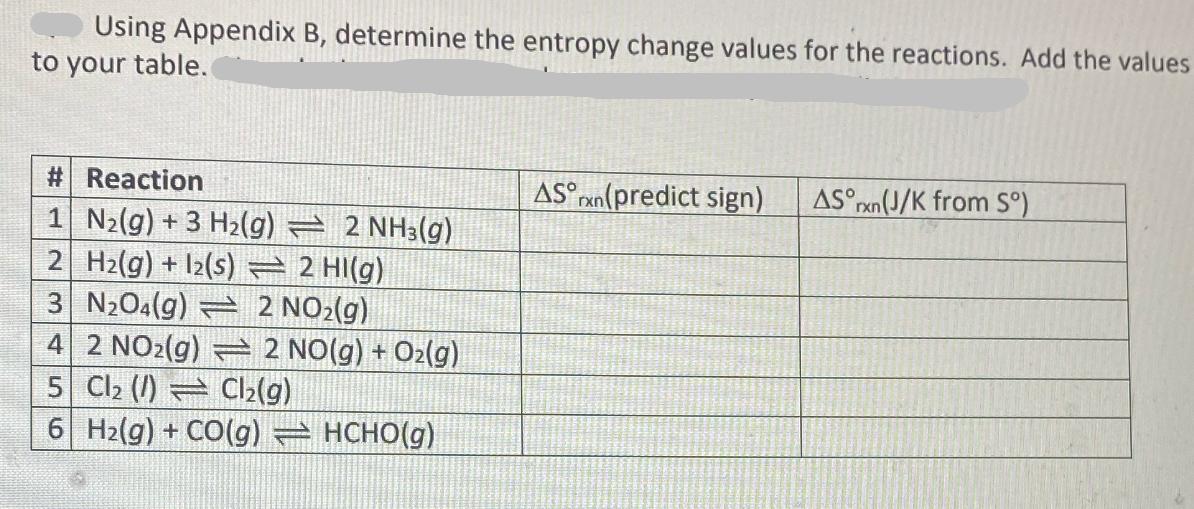
Using Appendix B, determine the entropy change values for the reactions. Add the values to your table. # Reaction 1 N2(g) +3 H2(g) AS (predict sign) AS rxn(J/K from S) 2 NH3(g) 2 H2(g) +12(s) 2 HI(g) 3 N2O4(g) 2 NO2(g) 42 NO2(g) 2 NO(g) + O2(g) 5 Cl (1) Cl2(g) 6| H2(g) + CO(g) = HCHO(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


