Question: Q2: Draw Lewis structures for all reactants and products, then calculate reaction enthalpy from bond energies (table 9.2 below). How do they compare? AHrxn
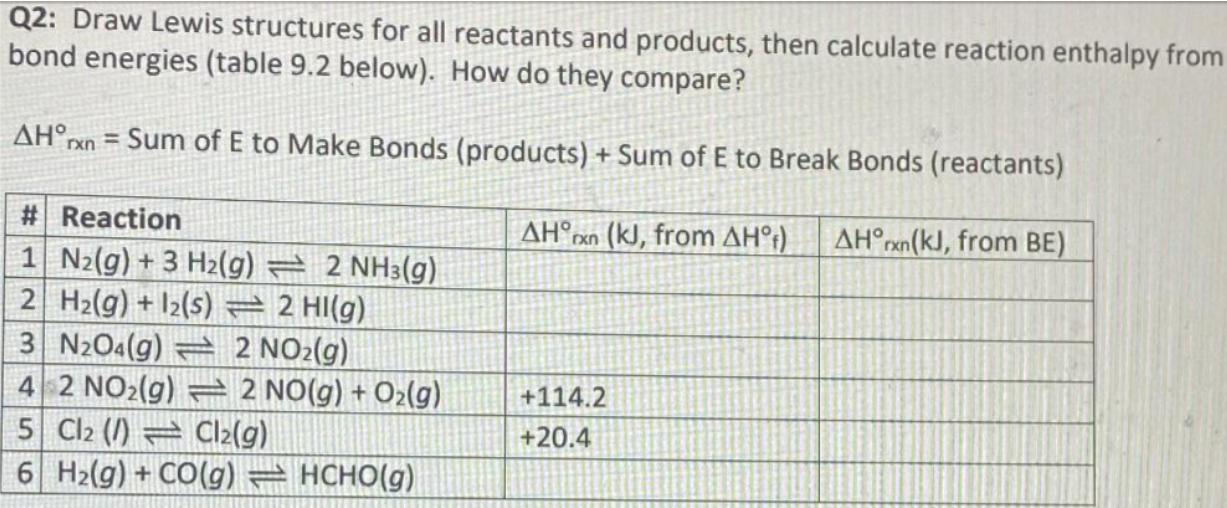
Q2: Draw Lewis structures for all reactants and products, then calculate reaction enthalpy from bond energies (table 9.2 below). How do they compare? AHrxn = Sum of E to Make Bonds (products) + Sum of E to Break Bonds (reactants) # Reaction AHxn (kJ, from AH) AHrxn(kJ, from BE) 1 N2(g) +3 H2(g) 2 NH3(g) 2 H2(g) +12(s) 2 HI(g) 3 N2O4(g) 2 NO2(g) 4 2 NO2(g) 2 NO(g) + O2(g) +114.2 5 Cl2 () Cl2(g) +20.4 6 Hz(g) + CO(g) = HCHO(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


