Question: using lechateliers Nitrogen monoxide and ozone react to form nitrogen dioxide and oxygen, like this: NO(g)+O3(g)NO2(g)+O2(g) The reaction is exothermic. Suppose a mixture of NO,O3,NO2
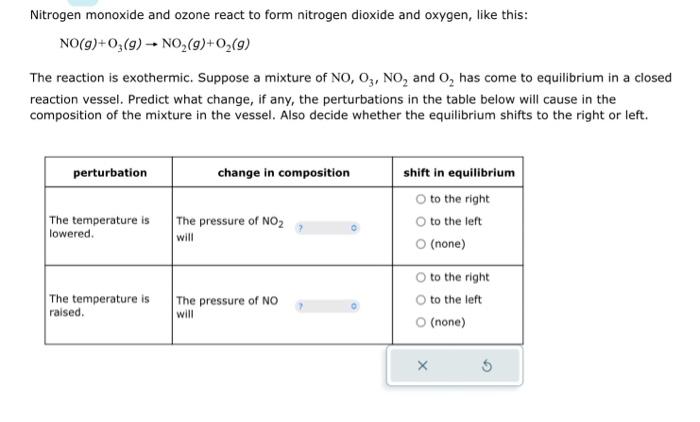
Nitrogen monoxide and ozone react to form nitrogen dioxide and oxygen, like this: NO(g)+O3(g)NO2(g)+O2(g) The reaction is exothermic. Suppose a mixture of NO,O3,NO2 and O2 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


