Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #15 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #15!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
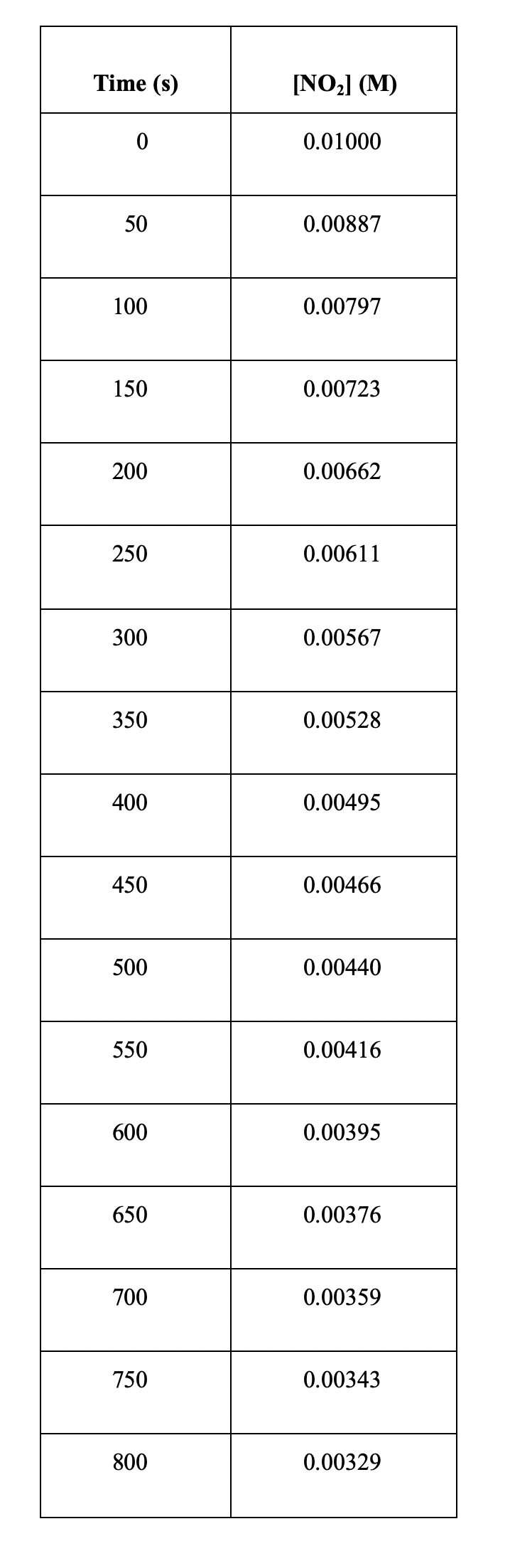

Table 2. Concentration versus time data for the decomposition of nitrogen dioxide, NO2, into nitrogen monoxide, NO, and oxygen, O2 15A. Use graphical analysis to determine the order of the reaction with respect to nitrogen dioxide. The data are already provided for you in the Google Sheet M6D1, supplemental. Explain how you determined the order of the reaction. Insert your work image here. 15B. Based on your results from 15A, write the rate law for this reaction and determine the rate constant, k. Type your answer here. 15C. Use your graph and the best-fit line (trendline) to estimate the concentration of nitrogen dioxide at 2000s. Insert your work image here. Table 2. Concentration versus time data for the decomposition of nitrogen dioxide, NO2, into nitrogen monoxide, NO, and oxygen, O2 15A. Use graphical analysis to determine the order of the reaction with respect to nitrogen dioxide. The data are already provided for you in the Google Sheet M6D1, supplemental. Explain how you determined the order of the reaction. Insert your work image here. 15B. Based on your results from 15A, write the rate law for this reaction and determine the rate constant, k. Type your answer here. 15C. Use your graph and the best-fit line (trendline) to estimate the concentration of nitrogen dioxide at 2000s. Insert your work image here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


