Question: Using the A-B binary equilibrium diagram given above; a) Calculate the activity coefficients and activity of A and B at the saturation limits at 800K.
Using the A-B binary equilibrium diagram given above; a) Calculate the activity coefficients and activity of A and B at the saturation limits at 800K. Draw the activity-composition diagram. b) Calculate the mixture free energy at the saturation limits at 800K and plot the composition-free energy change. c) Calculate the activity of B for XA=0.03 at 800 K. d) Calculate the interatomic bond parameter for XA=0.03 at 800 K. e) Calculate the activity of A for XA=0.03 at 800 K. f) Calculate the total free energy value of the solution for XA=0.03 at 800 K. g) Calculate the vapor pressure of A in solution for t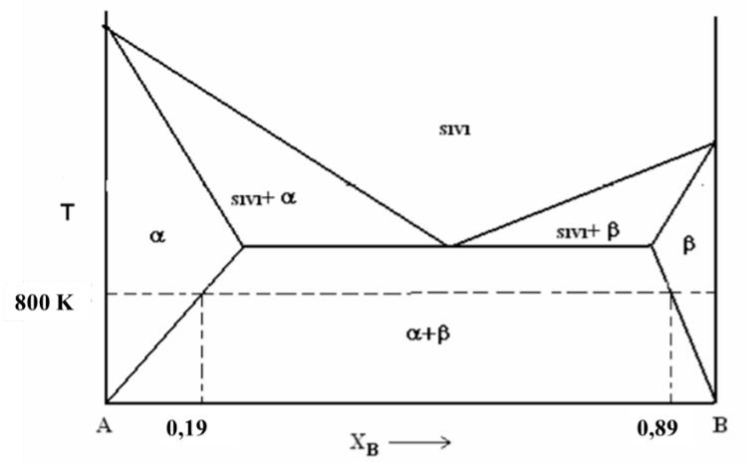 he composition XA=0.2 at 800 K.\( \operatorname{In} P_{A}^{0}=-\frac{33440}{T}-5,06 \operatorname{In} T+48,27 \)
he composition XA=0.2 at 800 K.\( \operatorname{In} P_{A}^{0}=-\frac{33440}{T}-5,06 \operatorname{In} T+48,27 \)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


