Question: Using the A-B binary equilibrium diagram given above; a) Calculate the activity coefficients and activity of A and B at the saturation limits at 800K.
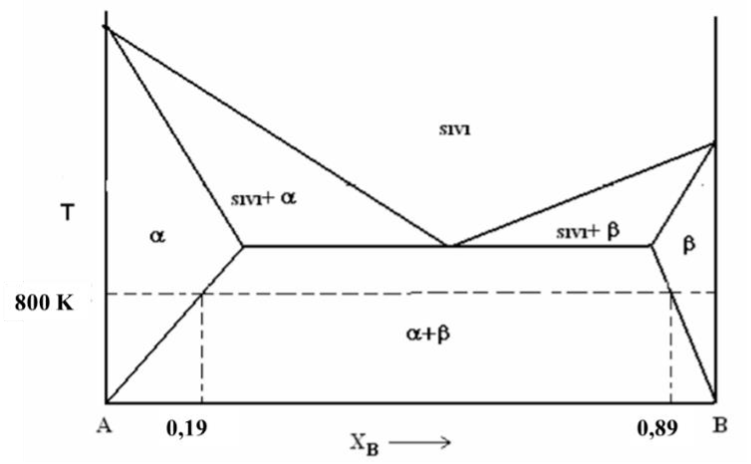
Using the A-B binary equilibrium diagram given above;
a) Calculate the activity coefficients and activity of A and B at the saturation limits at 800K. Draw the activity-composition diagram.
b) Calculate the mixture free energy at the saturation limits at 800K and plot the composition-free energy change.
c) Calculate the activity of B for XA=0.03 at 800 K.
d) Calculate the interatomic bond parameter for XA=0.03 at 800 K.
e) Calculate the activity of A for XA=0.03 at 800 K.
f) Calculate the total free energy value of the solution for XA=0.03 at 800 K.
g) Calculate the vapor pressure of A in solution for the composition XA=0.2 at 800 K.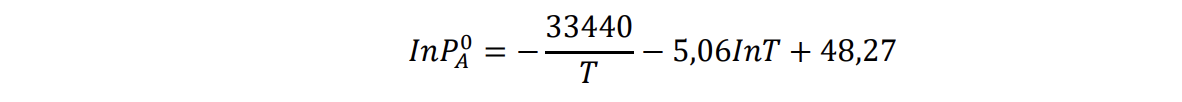
InPA0=T334405,06InT+48,27
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


