Question: Using the Al-Cu phase diagram provided, calculate the overall composition of the alloy and plot it as a vertical line. Answer the following questions: 1.
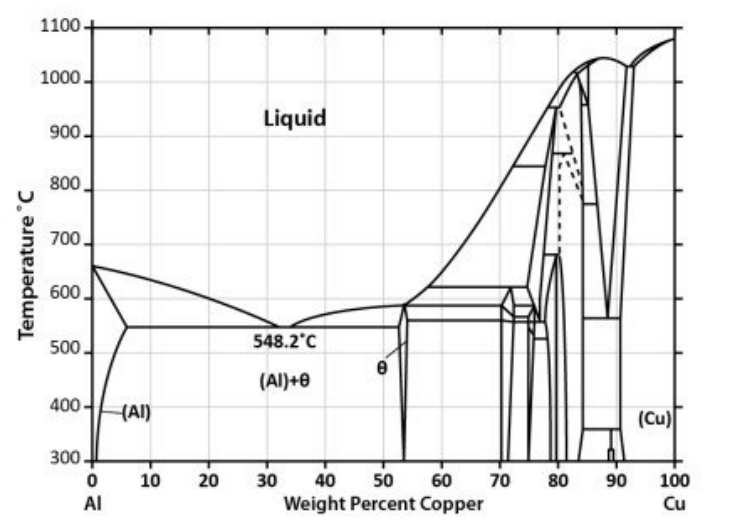
Using the Al-Cu phase diagram provided, calculate the overall composition of the alloy and plot it as a vertical line. Answer the following questions:
1. Is this alloy hypoeutectic or hypereutectic?
2. What is the atomic fraction of copper in this alloy?
3. At what temperature should the first solid appear?
4. What is the first solid to appear and what is its composition?
5. What is the second solid to appear and what is its composition?
6. If this alloy were reheated, what would be the composition of the first liquid to appear?
7. Estimate the shape of a plot of temperature vs. time for the cooling process from 1000C to room temperature. Explain the features of this cooling curve with reference to the phase diagram, including any curvature or changes in slope.
8. Offer an explanation as to why neither pure metal shows substantial solid solubility of the other. Also, why might the solubility of Al in Cu at 300C be somewhat higher than that of Cu in Al at the same temperature?
NOTE : initial mass of Al= 94.14g and initial mass of Copper = 21.52 g
1100 1000 Liquid 900 800 700 Temperature 600 500 548.2"c (AI)+0 400 (AI) (Cu) 300 10 20 80 90 20 30 40 50 60 70 Weight Percent Copper 100 Cu
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


