Question: Using the critical point, PC = 80.0 bar and TC = 512 K, what is the vapor pressure of methanol at 375 K? (Answer in
Using the critical point, PC = 80.0 bar and TC = 512 K, what is the vapor pressure of methanol at 375 K? (Answer in bar.)
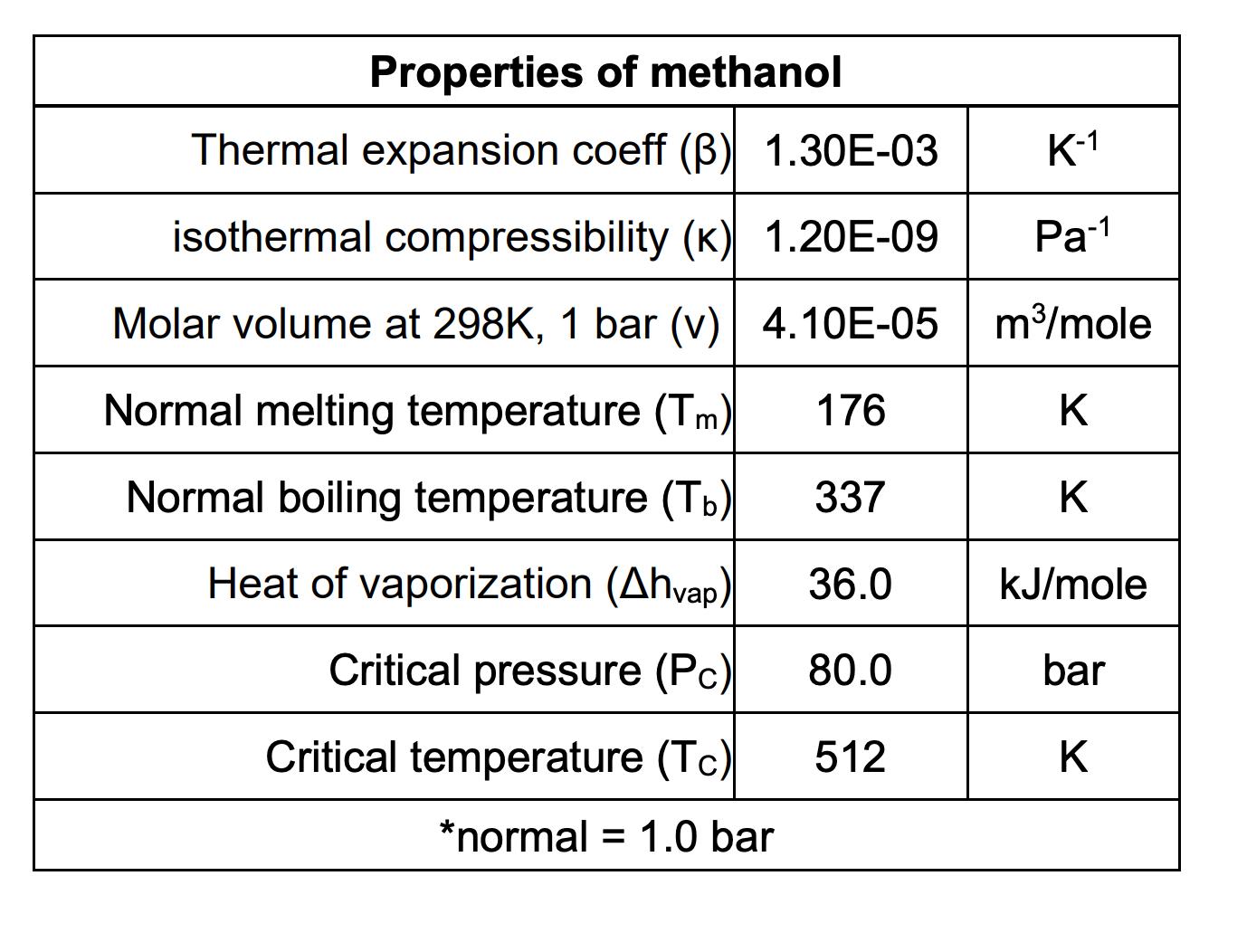
Properties of methanol Thermal expansion coeff () 1.30E-03 isothermal compressibility (K) 1.20E-09 Molar volume at 298K, 1 bar (v) 4.10E-05 K-1 Pa-1 m/mole Normal melting temperature (Tm) 176 K Normal boiling temperature (Tb) 337 K Heat of vaporization (Ahvap) 36.0 kJ/mole Critical pressure (Pc) 80.0 bar Critical temperature (Tc) 512 K *normal = 1.0 bar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


