Question: Using the electronegativity values given in the references section, show for the indicated bonds which atom acquires the partial negative charge. (To answer this question
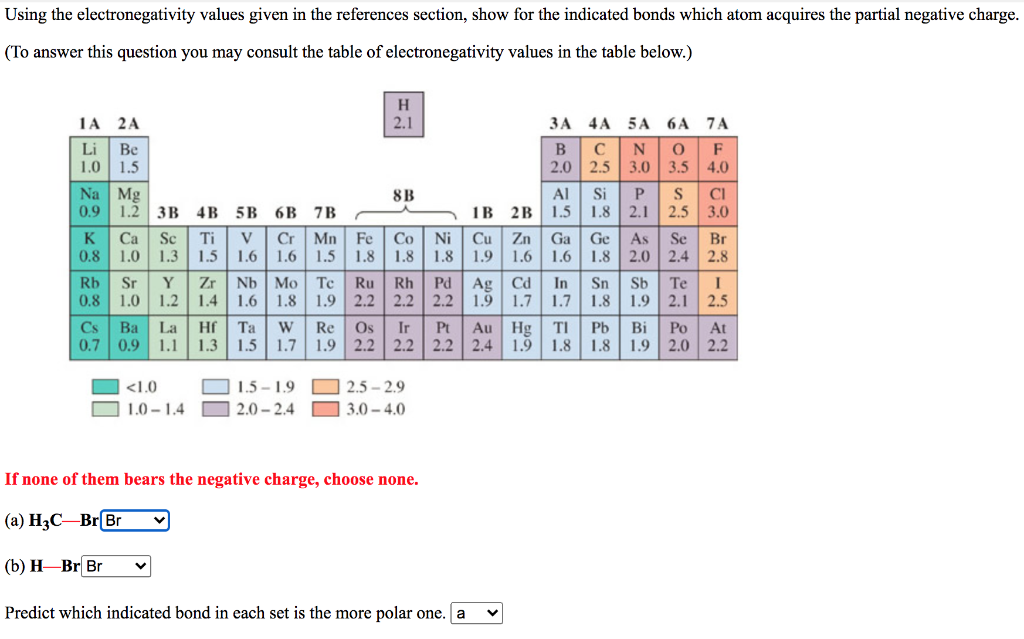
Using the electronegativity values given in the references section, show for the indicated bonds which atom acquires the partial negative charge. (To answer this question you may consult the table of electronegativity values in the table below.) H 1A 2A 2.1 4A 5 A 6A 7A Li Be B N O F 1.0 1.5 2.0 2.5 3.0 3.5 4.0 Na Mg 8B Si P S 0.9 1.2 3B 4B 5B 6B 7B 1B 2B 1.5 1.8 2.1 2.5 3.0 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 0.8 1.0 1.3 1.5 1.6 1.6 1.5 1.8 1.8 1.8 1.9 1.6 1.6 1.8 2.0 2.4 2.8 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 1 0.8 1.0 1.2 1.4 1.6 1.8 1.9 2.2 2.2 2.2 1.9 1.7 1.7 1.8 1.9 2.1 2.5 Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Ti Pb Bi Po At 0.7 0.9 1.1 1.3 1.5 1.7 1.9 2.2 2.2 2.2 2.4 1.9 1.8 1.8 1.9 2.0 2.2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


