Question: Using the following experimental time-concentration data obtained from a liquid-phase reaction of reactant A converting into products in a batch reactor with a constant volume,
Using the following experimental time-concentration data obtained from a liquid-phase reaction of reactant A converting into products in a batch reactor with a constant volume, determine the reaction rate constant (k) and reaction order (n). If the reaction were to be carried out in a CSTR reactor, what would be the necessary reactor volume for 80% conversion at a flow rate of 10 mol/s? 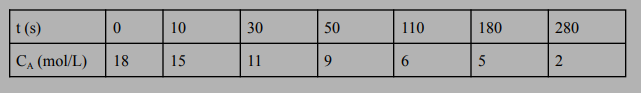
\begin{tabular}{|l|l|l|l|l|l|l|l|} \hline t(s) & 0 & 10 & 30 & 50 & 110 & 180 & 280 \\ \hline CA(mol/L) & 18 & 15 & 11 & 9 & 6 & 5 & 2 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


