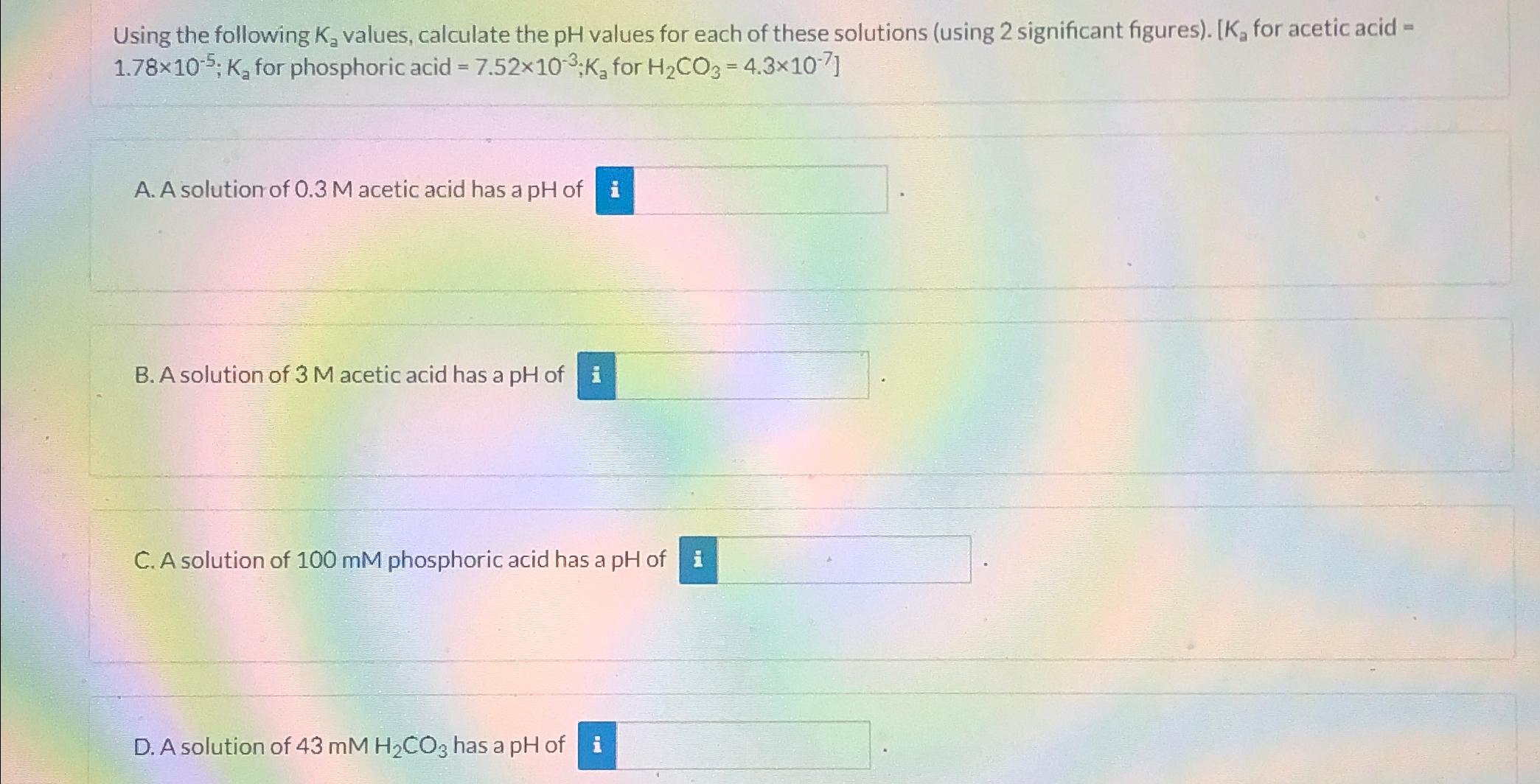
Question: Using the following K_(a) values, calculate the pH values for each of these solutions (using 2 significant figures). [ K_(a) for acetic acid = 1.78times
Using the following
K_(a)values, calculate the
pHvalues for each of these solutions (using 2 significant figures). [
K_(a)for acetic acid =
1.78\\\\times 10^(-5);K_(a)for phosphoric acid
=7.52\\\\times 10^(-3);K_(a)for
{[
:H_(2)CO_(3)=4.3\\\\times 10^(-7)]}\ A. A solution of
0.3Macetic acid has a
pHof\ B. A solution of
3Macetic acid has a
pHof\ C. A solution of
100mMphosphoric acid has a
pHof\ D. A solution of
43mMH_(2)CO_(3)has a pH of
Using the following Ka values, calculate the pH values for each of these solutions (using 2 significant figures). [ Ka for acetic acid = 1.78105;Ka for phosphoric acid =7.52103;Kaa for H2CO3=4.3107 ] A. A solution of 0.3M acetic acid has a pH of B. A solution of 3M acetic acid has a pH of C. A solution of 100mM phosphoric acid has a pH of D. A solution of 43mMH2CO3 has a pH of
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


