Question: Using the Given Data Do The Following: 1. Give one Example of Converting Absorbance to Percent Transmittance 2. Determine x, y, and k for the
Using the Given Data Do The Following: 1. Give one Example of Converting Absorbance to Percent Transmittance 2. Determine x, y, and k for the Equation: = [+] x [-] y using the modified equation: Rate = k' [crystV+]x where k' = k [OH-]y 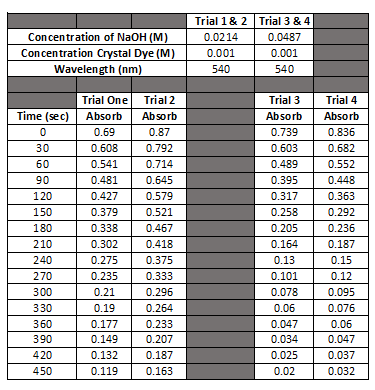
Concentration of NaOH (M) Concentration Crystal Dye (M) Wavelength (nm) Trial 1 & 2 Trial 3 & 4 0.0214 0.0487 0.001 0.001 540 540 Time (sec) 0 30 60 90 120 150 180 210 240 270 300 330 360 390 420 450 Trial One Absorb 0.69 0.608 0.541 0.481 0.427 0.379 0.338 0.302 0.275 0.235 0.21 0.19 0.177 0.149 0.132 0.119 Trial 2 Absorb 0.87 0.792 0.714 0.645 0.579 0.521 0.467 0.418 0.375 0.333 0.296 0.264 0.233 0.207 0.187 0.163 Trial 3 Absorb 0.739 0.603 0.489 0.395 0.317 0.258 0.205 0.164 0.13 0.101 0.078 0.06 0.047 0.034 0.025 0.02 Trial 4 Absorb 0.836 0.682 0.552 0.448 0.363 0.292 0.236 0.187 0.15 0.12 0.095 0.076 0.06 0.047 0.037 0.032
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


