Question: Using the phase diagram below as a guide, a substance that is at low temperature and high pressure is most likely to be a Phase
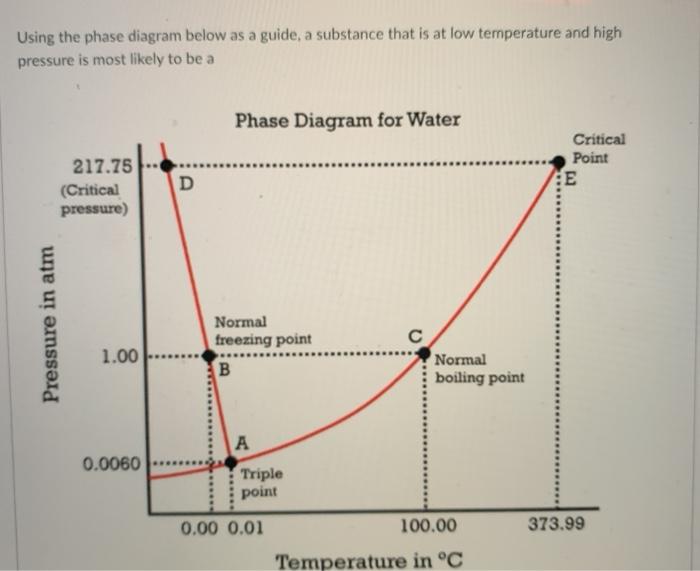
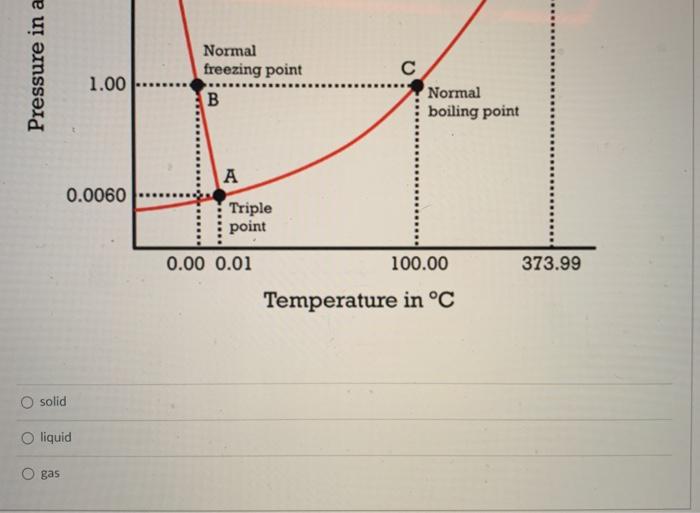
Using the phase diagram below as a guide, a substance that is at low temperature and high pressure is most likely to be a Phase Diagram for Water Critical Point E 217.75 (Critical pressure) D Pressure in atm Normal freezing point B 1.00 Normal boiling point 0.0060 Triple point 0.00 0.01 100.00 373.99 Temperature in C Pressure in a 1.00 Normal freezing point B Normal boiling point 0.0060 Triple point 0.00 0.01 373.99 100.00 Temperature in C solid O liquid o gas Question 18 5 pts The process of freezing is Select] and the sign of AH is Select 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


