Question: Using the phase diagram at right for an unknown substance, what is the name of the phase change is occurring when pressure and temperature
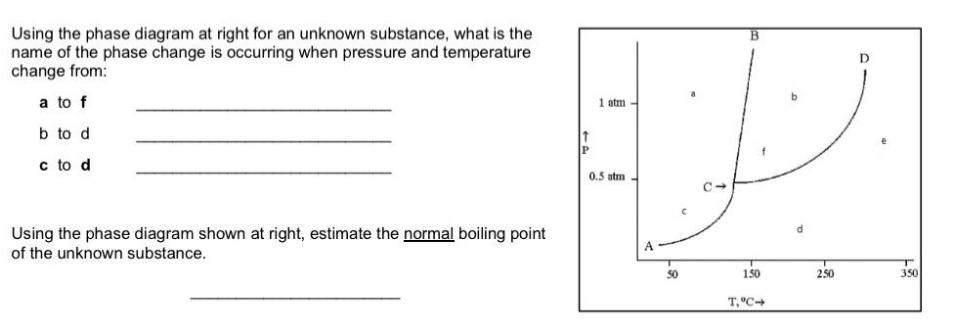
Using the phase diagram at right for an unknown substance, what is the name of the phase change is occurring when pressure and temperature change from: a to f b to d c to d Using the phase diagram shown at right, estimate the normal boiling point of the unknown substance. P 1 atm 0.5 atm - 50 B 150 T, C- d 2.50 D 350
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it
The image displays a phase diagram that represents the different phases solid liquid and gas of a su... View full answer

Get step-by-step solutions from verified subject matter experts


