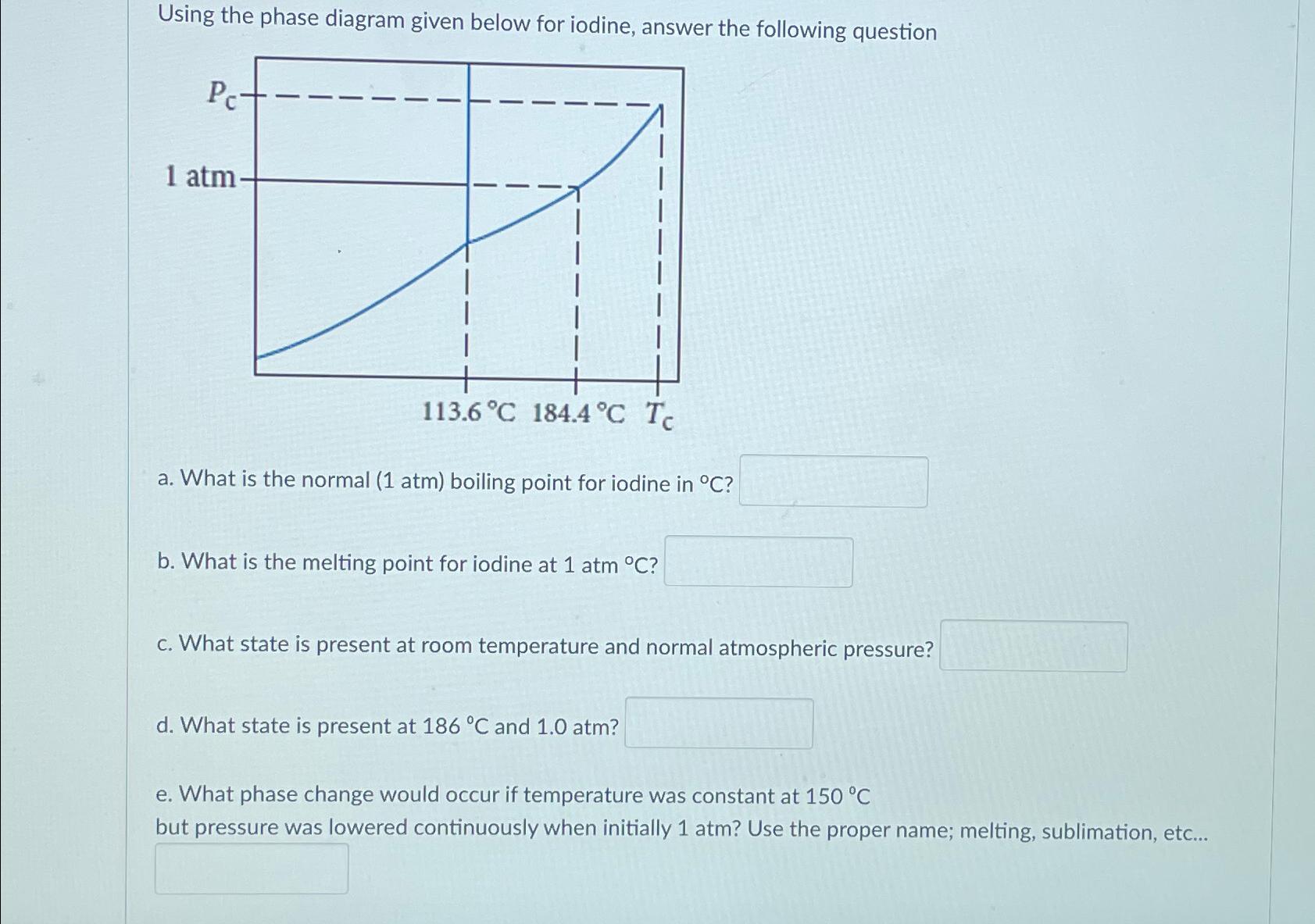
Question: Using the phase diagram given below for iodine, answer the following question a . What is the normal ( 1 atm ) boiling point for
Using the phase diagram given below for iodine, answer the following question
a What is the normal atm boiling point for iodine in
b What is the melting point for iodine at atm
c What state is present at room temperature and normal atmospheric pressure?
d What state is present at and atm
e What phase change would occur if temperature was constant at but pressure was lowered continuously when initially atm? Use the proper name; melting, sublimation, etc...
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


