Question: Using the root mean square spoed, V. (v)!= 36T/m.calculate the gas temperature of He for which : -0.25 nm, a typical value needed to resolve

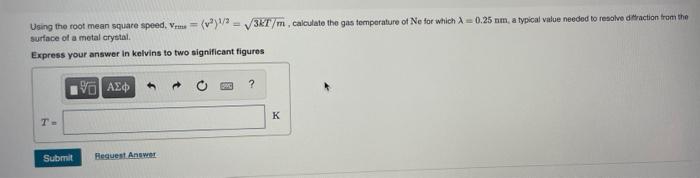
Using the root mean square spoed, V. (v)!= 36T/m.calculate the gas temperature of He for which : -0.25 nm, a typical value needed to resolve diffraction from the surface of a motal crystal Express your answer in kelvins to two significant figures ] ? T. K Submit Request Answer Using the root mean square speed. Vr = {v}/2 36T/m, calculate the gas temperature of Ne for which 2 = 0.25 nm, a typical value needed to resolve direction from the surface of a metal crystal Express your answer in kelvins to two significant figures ? T- Submit Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


