Question: Using the same arguments that were used to derive the Langmuir isotherm, instead derive a 2-layer Langmuir isotherm (see the Figure below). You should assume
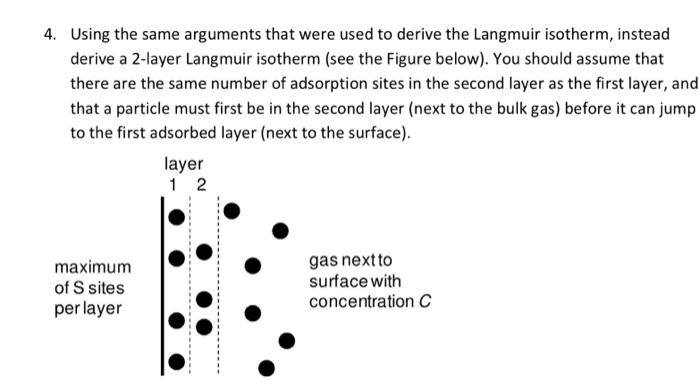
Using the same arguments that were used to derive the Langmuir isotherm, instead derive a 2-layer Langmuir isotherm (see the Figure below). You should assume that there are the same number of adsorption sites in the second layer as the first layer, and that a particle must first be in the second layer (next to the bulk gas) before it can jump to the first adsorbed layer (next to the surface)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


