Using the single-step global mechanism for combustion of a hydrocarbon with air, compare the mass rates of
Fantastic news! We've Found the answer you've been seeking!
Question:
Using the single-step global mechanism for combustion of a hydrocarbon with air,
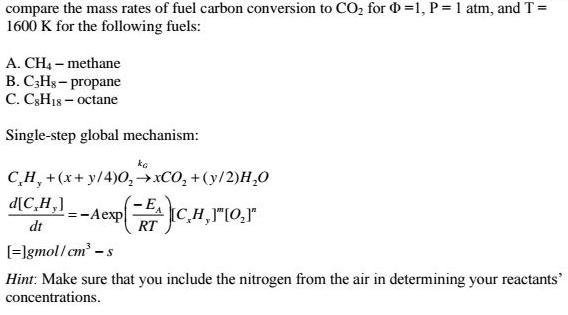
Related Book For 

Posted Date:




