Question: Using the viscosity values given as a function of temperature for glycerin, a) determine the activation energy as a function of viscosity.(E) b) find the
Using the viscosity values given as a function of temperature for glycerin,
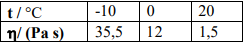
a) determine the activation energy as a function of viscosity.(E)
b) find the viscosity value at 15C.
\begin{tabular}{|l|l|l|l|} \hline t/C & -10 & 0 & 20 \\ \hline / (Pa s) & 35,5 & 12 & 1,5 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


