Question: Using values from the table of standard reduction potentials, calculate the standard free energy change (in kJ) for the following cells. (a) Cr(s) Cr** (aq)
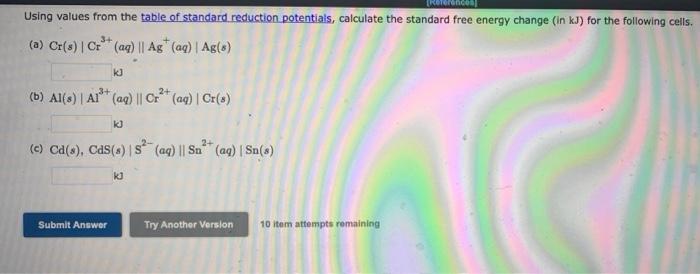
Using values from the table of standard reduction potentials, calculate the standard free energy change (in kJ) for the following cells. (a) Cr(s) Cr** (aq) || Ag (aq) | Ag(s) 3+ KJ (b) Al(s) A!'* (aq) || Cr2+ (aq) | Cr(s) k] 2+ (c) Ca(), Cas() s? (aq) || Sm+ (aq) | Sn(s) KU Submit Answer Try Another Version 10 itam attempts remaining Using values from the table of standard reduction potentials, calculate the standard free energy change (in kJ) for the following cells. (a) Cr(s) Cr** (aq) || Ag (aq) | Ag(s) 3+ KJ (b) Al(s) A!'* (aq) || Cr2+ (aq) | Cr(s) k] 2+ (c) Ca(), Cas() s? (aq) || Sm+ (aq) | Sn(s) KU Submit Answer Try Another Version 10 itam attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


