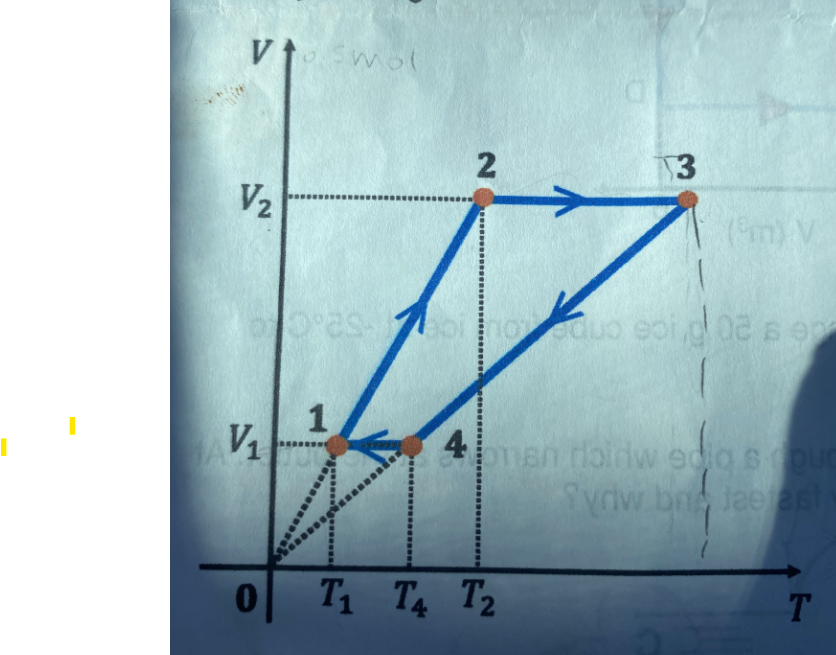
Question: v = 0 . 5 mol gas undergoes a transformation as shown in the figure below. Knowing T 1 = 3 0 0 K and
v mol gas undergoes a transformation as shown in the figure below. Knowing TK and TK vv E calculate the work done. Draw the PV and PT diagrams.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


