Question: V2 is 50ml the final volume is 50ml 9. (a) Calculate the theoretical pH of water after the addition of each portion of HC Show
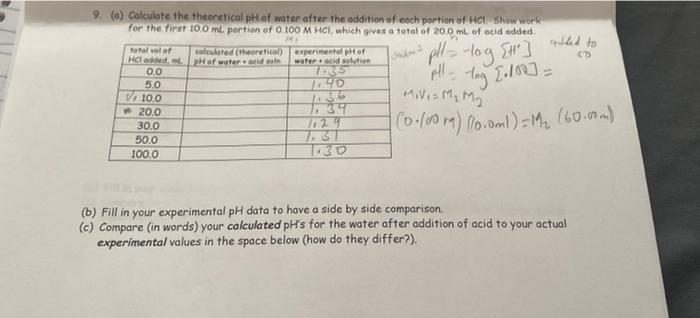
9. (a) Calculate the theoretical pH of water after the addition of each portion of HC Show work for the first 100 ml. portion of 0.100 M HCI, which gives a total of 20.0 ms of acid added Total valor calculated (theoretical experimental pH of He added, pH of water and son water acid solution 0.0 735 5.0 40 alled to ] - V/ 10.0 20.0 1 sudoma pH log [t] all-tog 1.100] = (0.00) locoml)- M 160.00m) Movi= M, M 2 30.0 50.0 1000 1129 TBT (b) Fill in your experimental pH data to have a side by side comparison (c) Compare (in words) your calculated pH's for the water after addition of acid to your actual experimental values in the space below (how do they differ?)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


