Question: values if needed for this question. Base/Stro. 2 1 pts 2req A 25.3 mL sample of 0.331 M methylamine, CH3NH2, is titrated with 0.224 M
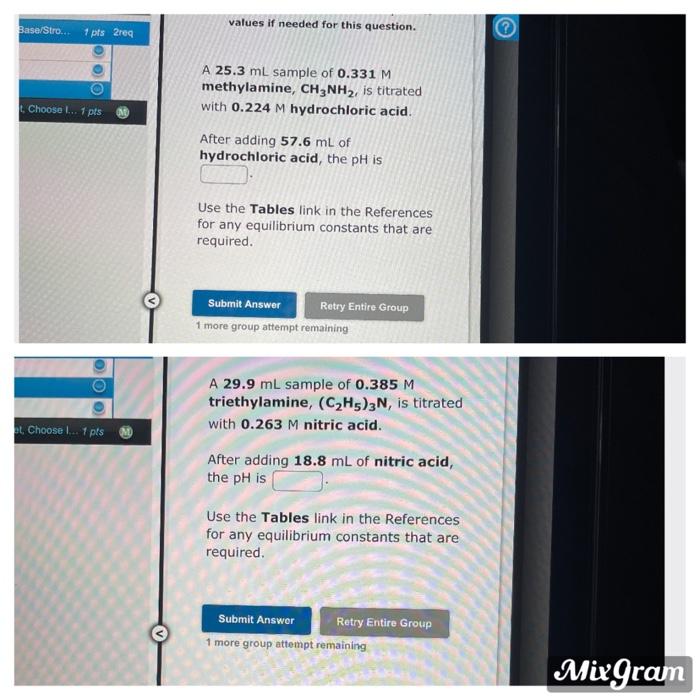
values if needed for this question. Base/Stro. 2 1 pts 2req A 25.3 mL sample of 0.331 M methylamine, CH3NH2, is titrated with 0.224 M hydrochloric acid. Choose L. 1 pts After adding 57.6 mL of hydrochloric acid, the pH is Use the Tables link in the References for any equilibrium constants that are required. Submit Answer Retry Entire Group 1 more group attempt remaining A 29.9 mL sample of 0.385 M triethylamine, (C2H5)3N, is titrated with 0.263 M nitric acid. B. Choose ... 1 pts After adding 18.8 mL of nitric acid, the pH is Use the Tables link in the References for any equilibrium constants that are required. Submit Answer Retry Entire Group 1 more group attempt remaining MixGram
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


