Question: values if needed for this question. ler the following reaction: 2HI(g)H2(g)+I2(g) If 4.21 moles of HI(g),0.698 moles of H2, and 0.580 moles of I2 are
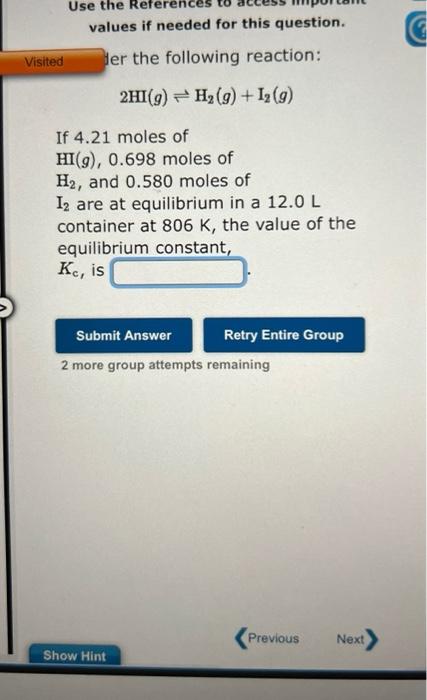
values if needed for this question. ler the following reaction: 2HI(g)H2(g)+I2(g) If 4.21 moles of HI(g),0.698 moles of H2, and 0.580 moles of I2 are at equilibrium in a 12.0L container at 806K, the value of the equilibrium constant, Kc, is 2 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


