Question: VAT Onte 1 Data Sheet Volumetric Analysis- Acid-Base Titration Concentration of Acetic Acid in Commercial sample of Vinegar 1. Brand name of vinegar sample: Avenca's
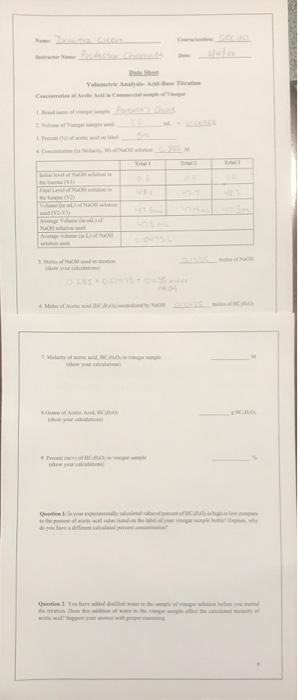
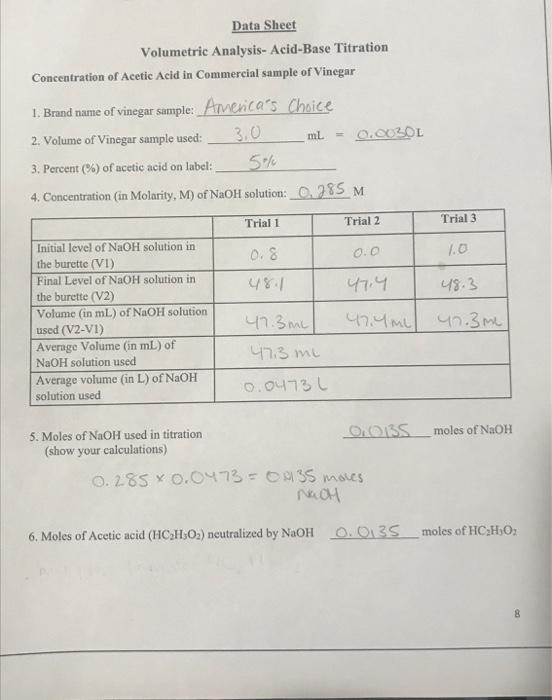

VAT Onte 1 Data Sheet Volumetric Analysis- Acid-Base Titration Concentration of Acetic Acid in Commercial sample of Vinegar 1. Brand name of vinegar sample: Avenca's Choice 2. Volume of Vinegar sample used: 3.0 ml = 0.0030L 3. Percent (%) of acetic acid on label: 5% 4. Concentration (in Molarity, M) of NaOH solution: 0.285 M Trial 1 Trial 2 Trial 3 0.8 0.0 1.0 47.4 48.3 Initial level of NaOH solution in the burette (VI) Final Level of NaOH solution in the burette (V2) Volume (in mL) of NaOH solution used (V2-V1) Average Volume (in mL) of NaOH solution used Average volume (in L) of NaOH solution used 47.4m 47.3me 47.3m 47:3 me 0.0473 moles of NaOH 5. Moles of NaOH used in titration OLOS (show your calculations) 0.285 x 0.0473 - 0435 moves Nach 6. Moles of Acetic acid (HCH,O2) neutralized by NaOH 0.0135 moles of HC:HO 8 M 7. Molarity of acetic acid, HC,H,O, in vinegar sample (show your calculations HC HOA 8. Grams of Acctic Acid, HC,H,O, (show your calculations 94 9. Percent (m/v) of HCH O. in vinegar sample (show your calculations) Question 1: Is your experimentally calculated value of percent of HC,H,O, is high or low compare to the percent of acetic acid value listed on the label of your vinegar sample bottle? Explain, why do you have a different calculated percent concentration? Question 2: You have added distilled water to the sample of vinegar solution before you started the titration. Does this addition of water to the vinegar sample affect the calculated molarity of acetic acid? Support your answer with proper reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


