Question: vity Readings Table 12.11 Sample PreLab - Predict Electrolyte type (strong/weakone) DI water nons Observation (none/dim/bright) Electrolyte type (strong weakone) Species in solution mone none
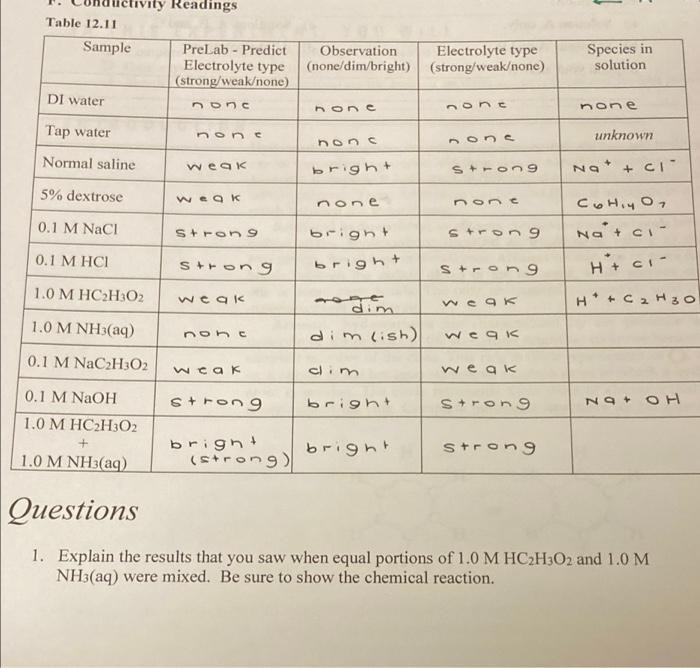
vity Readings Table 12.11 Sample PreLab - Predict Electrolyte type (strong/weakone) DI water nons Observation (none/dim/bright) Electrolyte type (strong weakone) Species in solution mone none none Tap water none one unknown non Normal saline weak bright Strong NO + CI 5% dextrose weak none non 0.1 M NaCl Strong Strong bright bright Cohiyo, Naci Htci 0.1 M HCI Strong Strong 1.0 M HC2H02 weak weak + + 1.0 M NH3(aq) modim dim lish) none weak 0.1 M NaC2H302 clim weak weak strong 0.1 M NaOH 1.0 M HC2H302 bright strong NaOH bright (strong bright strong 1.0 M NH3(aq) Questions 1. Explain the results that you saw when equal portions of 1.0 M HC2H3O2 and 1.0 M NH3(aq) were mixed. Be sure to show the chemical reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


