Question: Water needs to be removed from the previous stream, so it is passed through a condenser. Such as seen in the figure. Determine the mass
Water needs to be removed from the previous stream, so it is passed through a condenser. Such as seen in the figure. Determine the mass of water (kg/h) necessary to carry out the process (Use steam tables and assume that the pressure remains constant during the process.) The cp of the water liquid is 4.186 kJ/kg C. Additionally, assume that the non-condensable gases are not affected by the pressure.
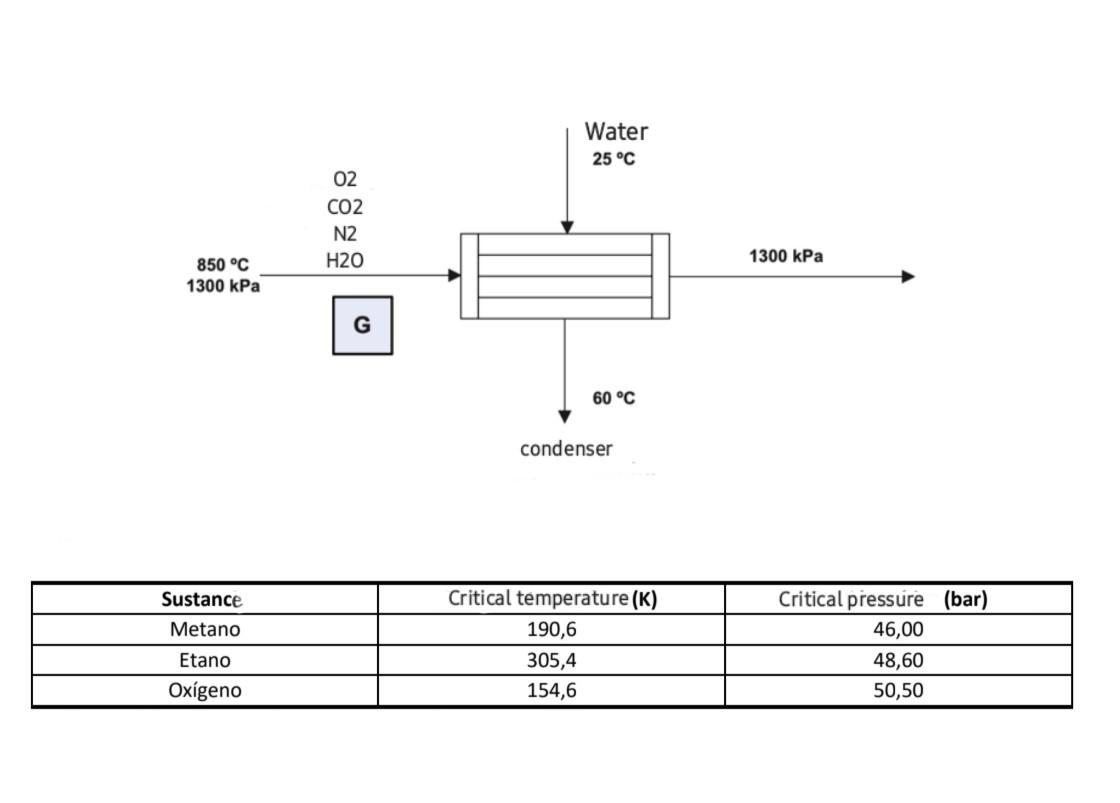
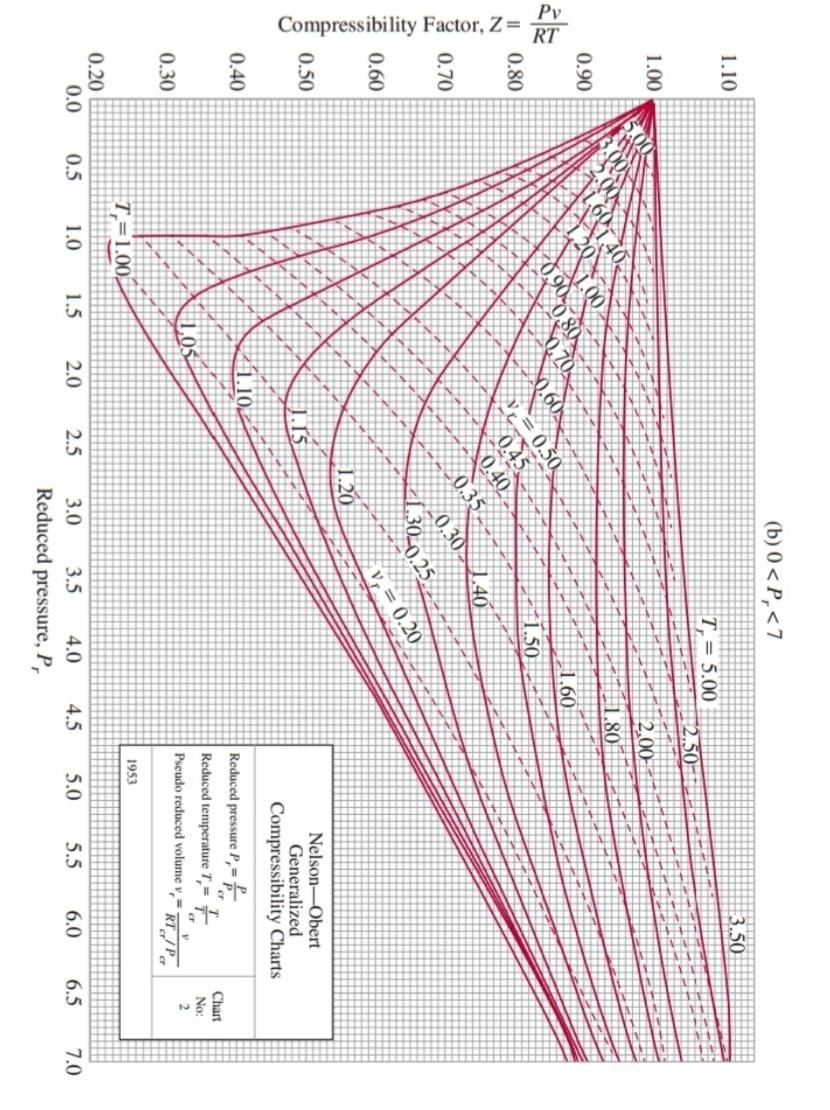
condenser \begin{tabular}{|c|c|c|} \hline Sustance & Critical temperature(K) & Critical pressure (bar) \\ \hline Metano & 190,6 & 46,00 \\ \hline Etano & 305,4 & 48,60 \\ \hline Oxgeno & 154,6 & 50,50 \\ \hline \end{tabular} Compressibility Factor, Z=RTPv
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


