Question: We used 0.03 g base in a sodium phosphate buffer(ph 7.4) and 100uM ANS from this how do we calculate the concentration . and the

 We used 0.03 g base in a sodium phosphate buffer(ph 7.4) and 100uM ANS from this how do we calculate the concentration . and the total BSA? we used 0.5 mL BSA for each solution, , like how do you determine r? Please help!! thank you
We used 0.03 g base in a sodium phosphate buffer(ph 7.4) and 100uM ANS from this how do we calculate the concentration . and the total BSA? we used 0.5 mL BSA for each solution, , like how do you determine r? Please help!! thank you
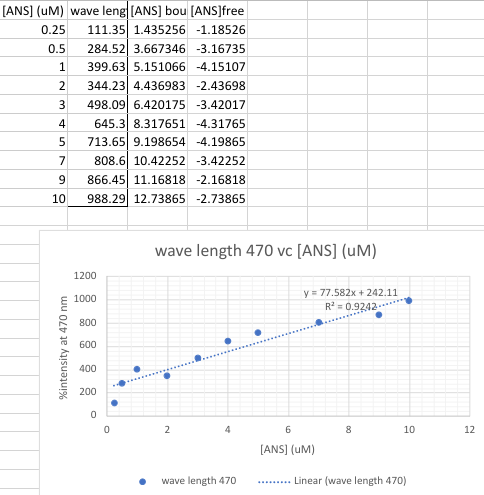
1. qalculate the BSA concentration 2. Calculated the ANS concentration 3. Show the linear plot and a sample calculation for determining (ANS)bound using the equation of the line 4. Calculate (ANS]free 5. Calculater 6. Finally calculate r/(ANS]free 7. Use the plot of r/[ANS]free vs r to determine the number of ANS bound per BSA and the Kd [ANS] (UM) wave leng[ANS] bou [ANS]free 0.25 111.35 1.435256 -1.18526 0.5 284.52 3.667346 -3.16735 1 399.63 5.151066 -4.15107 2 344.23 4.436983 -2.43698 3 498.09 6.420175 -3.42017 4 645.3 8.317651 -4.31765 5 713.65 9.198654 -4.19865 7 808.6 10.42252 -3.42252 9 866.45 11.16818 -2.16818 10 988.29 12.73865 -2.73865 an wave length 470 vc [ANS] (UM) 1200 1000 y = 77.582x + 242.11 R = 0.9242 800 %intensity at 470 nm 600 400 : 200 0 0 2 4 6 8 10 12 [ANS] (UM) wave length 470 ......... Linear (wave length 470)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


