Question: We will list the various quantities in the columns headed by the compound formulas. Let n be the number of moles of N2O4, and
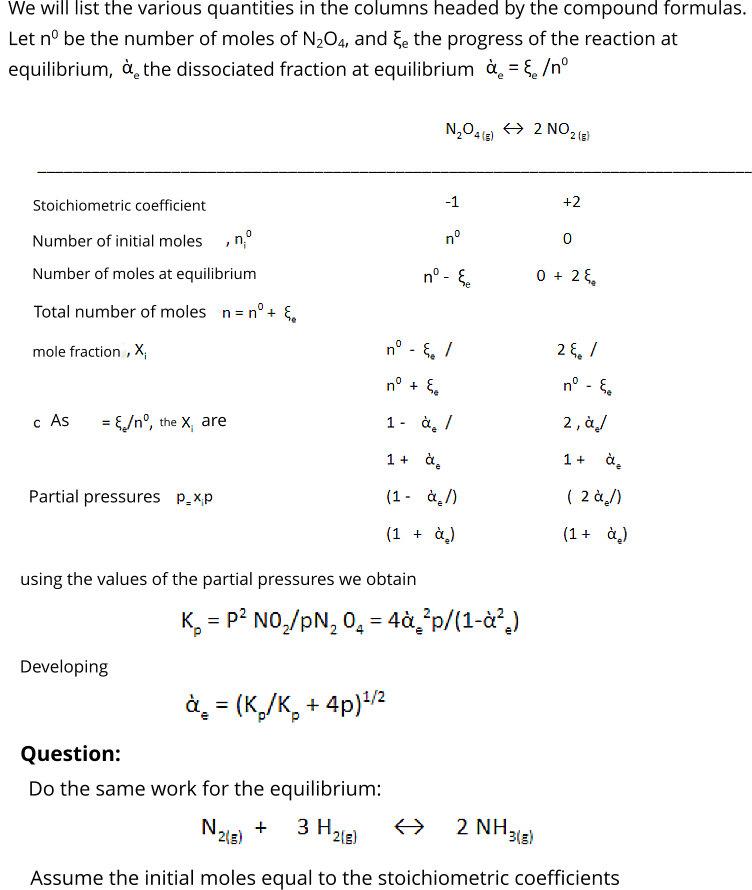
We will list the various quantities in the columns headed by the compound formulas. Let n be the number of moles of N2O4, and Ee the progress of the reaction at equilibrium, the dissociated fraction at equilibrium = /n %3D N,O4 + 2 NO,2(3) Stoichiometric coefficient -1 +2 Number of initial moles , n n Number of moles at equilibrium n - E. 0 + 2, De Total number of moles n= n + , mole fraction , X, n - , / 2 , / n + E, n - . c As = {/n, the X, are 1 - , / 2, / 1+ , 1 + . Partial pressures p.xp (1- ,/) ( 2 /) (1 + ) (1+ ) using the values of the partial pressures we obtain = p? NO,/pN, 0, = 4,'p/(1-,) Developing = (K,/K, + 4p)/2 Question: Do the same work for the equilibrium: + 2(g) 3 H218) 2 NH313) 2(g) 3(g) Assume the initial moles equal to the stoichiometric coefficients
Step by Step Solution
3.44 Rating (163 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


