Question: What is the approximate pka for the weak acid. pH 14 12- 10- 8 6 4 2 0 0 Answer: 2 (c) Equivalence point
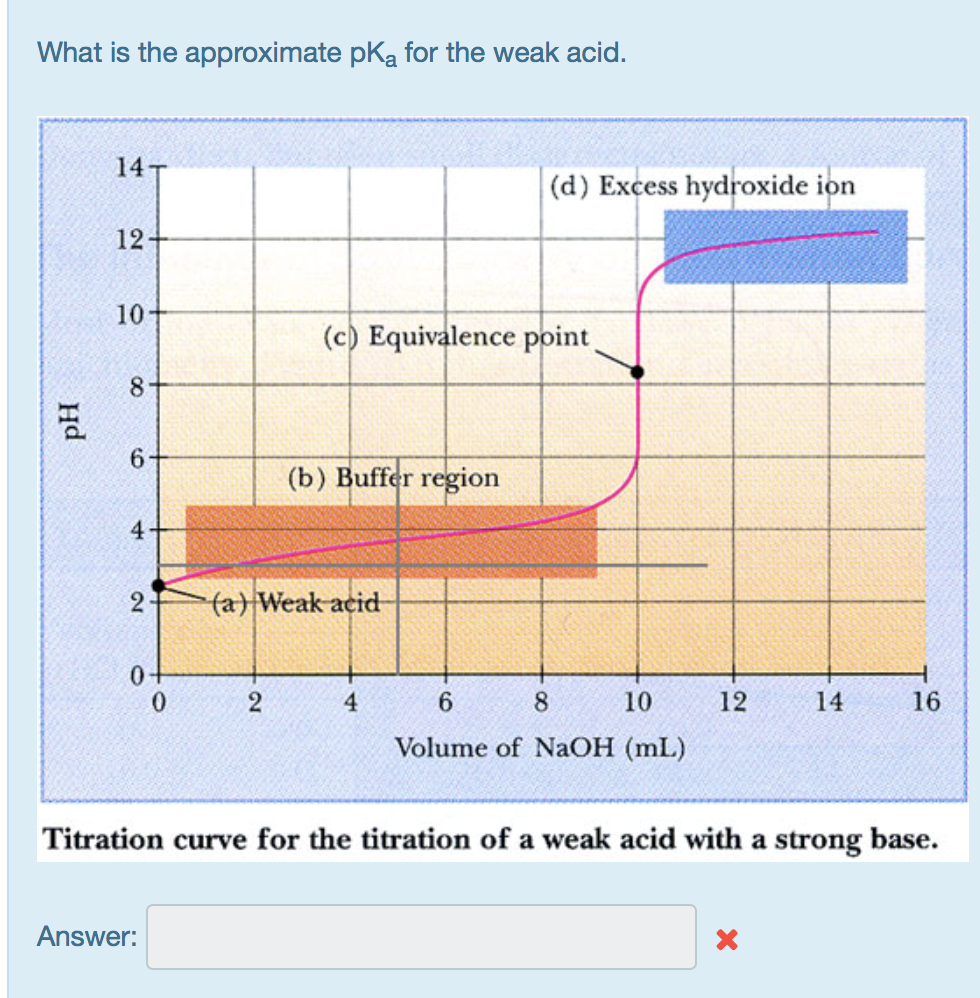
What is the approximate pka for the weak acid. pH 14 12- 10- 8 6 4 2 0 0 Answer: 2 (c) Equivalence point (b) Buffer region (a) Weak acid (d) Excess hydroxide ion 4 6 8 10 Volume of NaOH (mL) 12 14 Titration curve for the titration of a weak acid with a strong base. X 16
Step by Step Solution
★★★★★
3.49 Rating (156 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

pKa is the equal to pH at half equivalen... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


