Question
What is the required production rate of cumene for the following process? A company produces cumene (C9H12) using the process which) involves the reaction
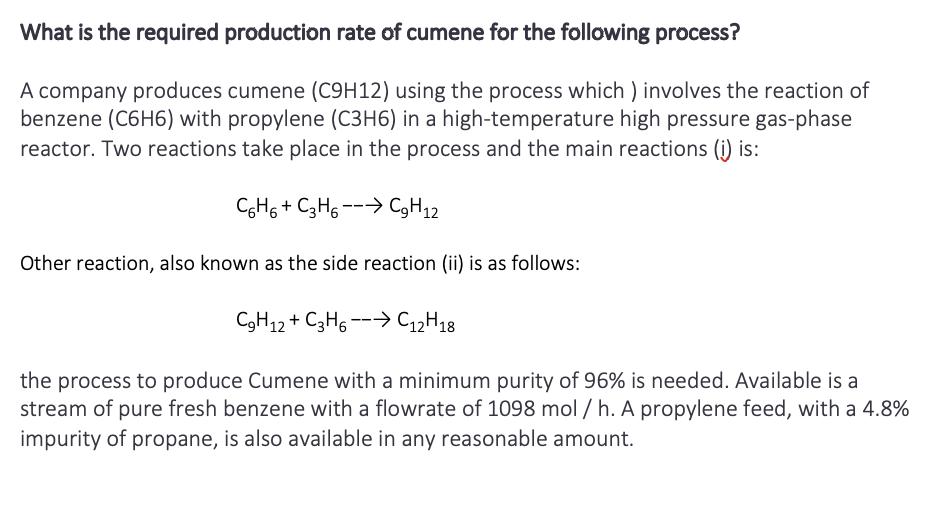
What is the required production rate of cumene for the following process? A company produces cumene (C9H12) using the process which) involves the reaction of benzene (C6H6) with propylene (C3H6) in a high-temperature high pressure gas-phase reactor. Two reactions take place in the process and the main reactions (i) is: C6H6+ C3H6C9H12 Other reaction, also known as the side reaction (ii) is as follows: C9H12+ C3H6C 12 H 18 the process to produce Cumene with a minimum purity of 96% is needed. Available is a stream of pure fresh benzene with a flowrate of 1098 mol / h. A propylene feed, with a 4.8% impurity of propane, is also available in any reasonable amount.
Step by Step Solution
3.31 Rating (118 Votes )
There are 3 Steps involved in it
Step: 1
The information provided allows us to calculate the minimum required production rate of cumene but n...
Get Instant Access with AI-Powered Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started